
HRP-47052
i
Human Research Program
Requirements Document
Verify this is the correct version before use
May 2017
Revision G, PCN 1
National Aeronautics and Space Administration
Lyndon B. Johnson Space Center
Houston, Texas
HRP-47052
ii
PREFACE
HUMAN RESEARCH PROGRAM REQUIREMENTS DOCUMENT
This document is the Human Research Program Requirements Document. The purpose of this
document is to define, document, and allocate Human Research Program (HRP) requirements.
The need to produce a Program Requirements Document (PRD) is established in HRP-47051,
Human Research Program – Program Plan, and is under configuration management control of
the Human Research Program Control Board (HRPCB).

HRP-47052
iii
Human Research Program
Requirements Document
Prepared By:
Original signature on file
________________________________________________
3/31/15
_______________
Maria Havenhill
Date
HRP Program Science Management Office Risk Manager
Human Research Program
Concurred By:
Original signature on file
3/31/15
Michele Perchonok
Date
Program Science Management Office Manager
Human Research Program
Approved By:
Original signature on file
________________________________________________
4/2/2015
_______________
William Paloski, Ph.D.
Date
Program Director
Human Research Program

HRP-47052
iv
DOCUMENT CHANGE/
REVISION LOG
PAGE 1 OF 1
Change/
Revision
Date Description of Change
Pages
Affected
Baseline
05-15-07
Initial Release (Reference per SLSDCR-HRPCB-
07-006, EFF. 05-15-07) approved by the HRPCB
All
Rev A
07-03-07
Revision (Reference per SLSDCR-HRPCB-07-
033, EFF. 07-03-07) approved outside-of-board
by the HRPCB chair.
Rev B
02-14-08
Revision (Reference per SLSDCR-HRPCB-08-
002, EFF. 02-14-08) approved by the HRPCB
Rev C
01-23-09
Revision (Reference per SLSDCR-HRPCB-08-
021, EFF. 01-23-09) approved by the HRPCB
Rev D
07-22-10
Revision (Reference per SLSDCR-HRPCB-10-
011, EFF. 07-22-10) approved by the HRPCB
Rev E
04-28-11
Revision (Reference per SLSDCR-HRPCB-11-
006, EFF. 04-28-11)
Rev E
05-19-11
Revision (Reference per SLSDCR-HRPCB-11-
006R1, Action Number AI-HRPCB-11-011, EFF.
05-19-11)
Rev E,
PCN-1
07-02-12
Page Change Notice Revision (Reference per
SLSD-HRPCB-12-013, EFF. 06-22-12)
Rev F
02-22-13
Revision (Reference per SLSDCR-HRPCB-12-
029-R1, EFF. 12-13-12)
Rev F,
PCN-1
08-20-13
Page Change Notice Revision (Reference per
SLSD-HRPCB-13-011, EFF. 08-20-13)
Rev G
03-12-15
Revision (Reference per HHPD-HRPCB-15-003,
EFF. 03-12-15)
Rev G,
PCN-1
05-17-17
Page Change Notice Revision (Reference per
HHPD-HRPCB-17-002, EFF. 04-10-17)

HRP-47052
v
TABLE OF CONTENTS
Section
Page
1 INTRODUCTION ............................................................................................ 1
1.1 Purpose ............................................................................................................................ 1
1.2 Scope ................................................................................................................................ 1
1.3 Responsibility and Change Authority .............................................................................. 2
2 DOCUMENTS .................................................................................................. 3
2.1 Applicable Documents ..................................................................................................... 3
2.2 Reference Documents ...................................................................................................... 3
3 HRP GOAL AND OBJECTIVES .................................................................. 5
3.1 Goal .................................................................................................................................. 5
3.2 Objectives ........................................................................................................................ 5
4 HRP REQUIREMENTS RELATED TO HUMAN SYSTEM
STANDARDS .......................................................................................................... 5
4.1 NASA’s Health, Medical, Human Performance, and Environmental Standards ............ 5
5 HRP REQUIREMENTS RELATED TO HH&P RISKS AND
CONCERNS ............................................................................................................ 6
5.1 Risk Assessment Methods ............................................................................................... 9
5.2 HRP Program Science Management Office (PSMO) Support ...................................... 10
5.3 Countermeasure and Technology Development ............................................................ 10
5.4 Development of Methods and Technologies to Monitor and Treat ............................... 11
5.5 Provision of Supporting Evidence ................................................................................. 11
6 HRP REQUIREMENTS RELATED TO ENABLING CAPABILITIES 12
6.1 Provision of Enabling Capabilities ................................................................................ 12
6.2 Preservation and Maintenance of Core Technical Capabilities and Expertise .............. 13
6.3 Compliance with Applicable Documents ...................................................................... 14
6.4 Optimization of Methods and Technologies .................................................................. 14
APPENDIX A: ACRONYMS AND ABBREVIATIONS ............................... 15

HRP-47052
vi
TABLE OF CONTENTS
Section
Page
APPENDIX B: DRM DESCRIPTIONS AND ASSUMPTIONS ................... 17
APPENDIX C: HUMAN RISK DISPOSITIONS FOR ALL DRMS ........... 31
LIST OF TABLES
Table
Page
Table 1 – Applicable Documents …………………………………………………………........3
Table 2 – Reference Documents …………………………………………………………….....4
Table 3 – Human System Health and Performance Risks Addressed by HRP ………………..8
Table 4 – Human System Health and Performance Concerns Addressed by HRP ……………9
LIST OF FIGURES
Figure
Page
Figure 1: HRP Management Architecture ……………………………………………………...7
Figure 2: HSRB DRM Categories …………………………………………………………….17
HRP-47052
1
1 INTRODUCTION
1.1 PURPOSE
This document defines, documents, and allocates the Human Research Program (HRP)
requirements to the HRP Program Elements. It also establishes the flow of requirements from
the Human Exploration and Operations Mission Directorate (HEOMD) and the Office of the
Chief Health and Medical Officer (OCHMO) down to the various HRP Program Elements to
ensure that human research and technology countermeasure investments support the delivery of
countermeasures and technologies that satisfy HEOMD’s and OCHMO’s exploration mission
requirements.
1.2 SCOPE
Requirements driving HRP work and deliverables are derived from the exploration architecture
as well as Agency standards regarding the maintenance of Human Health and Performance
(HH&P). Agency HH&P standards will define acceptable risks for each Design Reference
Mission (DRM) category as defined by the Human System Risk Board (HSRB). It is critical to
have the best available scientific, operational and clinical evidence in setting and validating these
standards. In addition, it is imperative that the best available evidence on preventing and
mitigating HH&P risks is incorporated into exploration mission and vehicle designs. These
elements form the basis of the HRP research and technology development requirements and
highlight the importance of HRP investments in enabling NASA’s exploration missions.
HRP requirements are derived from the following documents:
• Human Exploration and Operations Mission Directorate (HEOMD) Strategic
Implementation Plan;
• NPD 1001.0A, 2011 NASA Strategic Plan
• Human Exploration and Operations Mission Directorate (HEOMD) Program
Commitment Agreement (PCA) with HRP
• NASA-STD-3001, NASA Space Flight Human System Standard, Volume 1: Crew
Health; and
• NASA-STD-3001, NASA Space Flight Human System Standard, Volume 2: Human
Factors, Habitability and Environmental Health.
This PRD defines the requirements of the HRP which are allocated to the following Program
Elements:
1. Exploration Medical Capability (ExMC),
2. Human Factors and Behavioral Performance (HFBP),
3. Human Health Countermeasures (HHC),
4. ISS Medical Projects (ISSMP), and
5. Space Radiation (SR).
The requirements are subdivided into the following three categories:
1. Human system standards (section 4),
HRP-47052
2
2. Human health and performance risks (section 5), and
3. Provision of enabling capabilities (section 6).
Where appropriate, the Program Elements further allocate requirements to their research and
technology development portfolios. These allocations are documented in the Element Plans.
This document includes three appendices. Appendix A captures the acronyms used in this
document. Appendix B encompasses additional HRP assumptions on the DRM categories to
frame the research required for each of the HH&P risks in its portfolio. Appendix C is a matrix
of risk dispositions of all human spaceflight risks by DRM category and by in-mission/post-
mission phase.
1.3 RESPONSIBILITY AND CHANGE AUTHORITY
This document is under Configuration Management (CM) control of the HRPCB. Changes to
this document will result in the issuance of Page Change Notices (PCN) or a full re-issue of the
document using the Change Request (CR) process. A review of the PRD will be performed and
changes made as necessary to maintain consistency with the evolving HEOMD strategies, goals,
and objectives.

HRP-47052
3
2 DOCUMENTS
2.1 APPLICABLE DOCUMENTS
The following documents of the specified revision or the latest revision if not identified, are
applicable to the extent specified herein. Inclusion of applicable documents herein does not in
any way imply any order of precedence.
Table 1 – Applicable Documents
Document No.
Document Title
NASA-STD-3001 Vol. 1,
Rev A
NASA Space Flight Human System Standard Volume 1, Revision
A: Crew Health
NASA-STD-3001 Vol. 2,
Rev A
NASA Space Flight Human System Standard Volume 2: Human
Factors, Habitability and Environmental Health
NPD 1001.0B
2014 NASA Strategic Plan
HRP-XPCA
HEOMD Program Commitment Agreement (PCA) with HRP
HRP-47051B
Human Research Program – Program Plan (Revision B-PCN1)
NPR 7120.5E
NASA Space Flight Program and Project Management
Requirements w/Changes 1-15
NPD 1000.0B
NASA Governance and Strategic Management Handbook
NPD 8500.1C
NASA Environmental Management
NPD 8910.1B
Care and Use of Animals (Revalidated 6/25/13)
NPR 1080.1A
Requirements for the Conduct of NASA Research & Technology
(R&T)
NPR 2190.1B
NASA Export Control Program
NPR 2810.1A
Security of Information Technology (Revalidated with Change 1,
dated May 19, 2011)
NPR 5810.1
Standard Format for NASA Research Announcements (NRAs)
and other Announcements for Grants and Cooperative Agreements
(Updated w/Change 2, July 16, 2012).
NPR 7100.1
Protection of Human Research Subjects (Revalidated 6/26/14)
NPR 7120.8
NASA Research and Technology Program and Project
Management Requirements (w/Change 4 dated 01/04/17)
NID 8000.108
Agency Risk Management Procedural Requirements
NPR 7123.1B
NASA Systems Engineering Processes and Requirements
JSC 66705
Human System Risk Management Plan
2.2 REFERENCE DOCUMENTS
The following documents contain supplemental information to guide the user in the application
of this document. These reference documents may or may not be specifically cited within the
text of the document.

HRP-47052
4
Table 2 – Reference Documents
Document No.
Document Title
HRP-47053
Human Research Program Science Management Plan (Revision E
PCN-1)
HRP-47065
Human Research Program Integrated Research Plan (Revision H
PCN-3) (electronically available at:
http://humanresearchroadmap.nasa.gov/)
JSC-28330
Human Health and Performance Directorate Configuration
Management Plan (Revision F)
N/A
HRP Risk Evidence Reports electronically available at:
http://humanresearchroadmap.nasa.gov/evidence/
NPD 1000.3E
The NASA Organization w/Change 15
NPD 7100.8E
Protection of Human Research Subjects (Revalidated with admin.
changes 12/18/2012)
HRP-47069D
Human Research Program Unique Processes, Criteria, and
Guidelines (Revision D PCN-2)
S.1281
National Aeronautics and Space Administration (NASA)
Authorization Act of 2005
NASA/SP-2010-3407
Human Integration Design Handbook
N/A
NASA Institutional Review Board Website - http://irb.nasa.gov/
HRP-47052
5
3 HRP GOAL AND OBJECTIVES
This section reflects the HRP Goals and Objectives described in the HRP Program Commitment
Agreement and HRP-47051, Human Research Program – Program Plan.
3.1 GOAL
The goal of the HRP is to provide HH&P countermeasures, knowledge, technologies, and tools
to enable safe, reliable, and productive human space exploration.
3.2 OBJECTIVES
The specific objectives of the HRP are:
3.2.1 Develop capabilities, necessary countermeasures, and technologies in support of human
space exploration, focusing on mitigating the highest risks to crew health and
performance. Enable the definition and improvement of human spaceflight medical,
environmental and human factors standards.
3.2.2 Develop technologies that serve to reduce medical and environmental risks, to reduce
human systems resource requirements (mass, volume, power, data, etc.), and to ensure
effective human-system integration across exploration mission systems.
3.2.3 Ensure maintenance of Agency core competencies necessary to enable risk reduction in
the following areas: space medicine; physiological and behavioral effects of long-
duration spaceflight on the human body; space environmental effects (including
radiation) on human health and performance; and space human factors.
4 HRP REQUIREMENTS RELATED TO HUMAN SYSTEM
STANDARDS
4.1 NASA’S HEALTH, MEDICAL, HUMAN PERFORMANCE, AND
ENVIRONMENTAL STANDARDS
The HRP shall enable the development and modification of NASA’s health, medical, human
performance, and environmental standards in time for exploration mission planning and design.
Rationale: Spaceflight human system standards are designed to address
acceptable levels of HH&P risks for exploration missions of varying complexity
and duration. The OCHMO has established an initial set of standards that serves
to guide the HRP in the expansion of its evidence base regarding human
spaceflight health and performance risks. HRP sponsors research and technology
development enabling development and modification of the OCHMO maintained
standards.

HRP-47052
6
Several different types of standards have been established by the OCHMO and documented in
NASA-STD-3001, NASA Space Flight Human Systems Standards, Vol. 1 and Vol. 2. The
standards sets are listed below.
1. Fitness-for-duty standards for maintaining the physiological and behavioral parameters
necessary to perform the required tasks;
2. Permissible outcome limits for the changes in health outcomes that are potentially
affected by long-term exposure to the space environment;
3. Permissible exposure limits for managing risks by controlling human exposure;
4. Levels of care standards for guiding medical capabilities needed to respond to a medical
contingency during exploration missions; and
5. Human factors, habitability, and environmental standards to guide the development of
spacecraft and systems so as to alleviate human health and performance impacts.
HRP will perform the research required to recommend new standards where current standards do
not exist, or recommend updates to existing standards as evidence becomes available. The HRP
requirements necessary to ensure the best possible evidence base in order to enable the
development and modification of standards are the following:
4.1.1 The HHC shall perform the research necessary to enable the development and
modification of the HHC standards sets documented in NASA-STD-3001, Vol.1 and Vol.
2.
4.1.2 The HFBP shall perform the research necessary to enable the development and
modification of the HFBP standards sets documented in NASA-STD-3001, Vol.1 and
Vol. 2.
4.1.3 The SR shall perform the research necessary to enable the development and modification
of the SR standards sets documented in NASA-STD-3001, Vol.1 and Vol. 2.
4.1.4 The ExMC shall perform the research necessary to enable the development and
modification of the ExMC standards sets documented in NASA-STD-3001, Vol.1 and
Vol. 2.
5 HRP REQUIREMENTS RELATED TO HH&P RISKS AND
CONCERNS
A risk is a health and performance item of interest that has a clear consequence and attendant
likelihood supported by evidence. A concern is an item for which there is not sufficient evidence
or quantifiable likelihood for a given design reference mission to support its status as a risk.
Human System Risk Board
The OCHMO owns all HH&P risks managed by the HSRB as described in the JSC-66705,
Human System Risk Management Plan. The HSRB baselines risks supported by evidence and
determines which risks require research as part of the mitigation strategy. For concerns, the
HSRB makes the determination if more work is needed to seek out the minimum necessary
HRP-47052
7
spaceflight and terrestrial evidence to generate a likelihood and consequence (LxC) assessment.
If the HSRB determines research is required to understand a concern or risk, or mitigate a risk,
and if it is determined the research can be provided by HRP, the program will complete an
analysis of the risk or concern and develop a research plan to further understand the risk or
concern, inform standards, or develop mitigation or monitoring strategies for the risk. The HRP
records these risks and concerns as requirements in the PRD.
HRP Management Architecture to Address Risks and Concerns
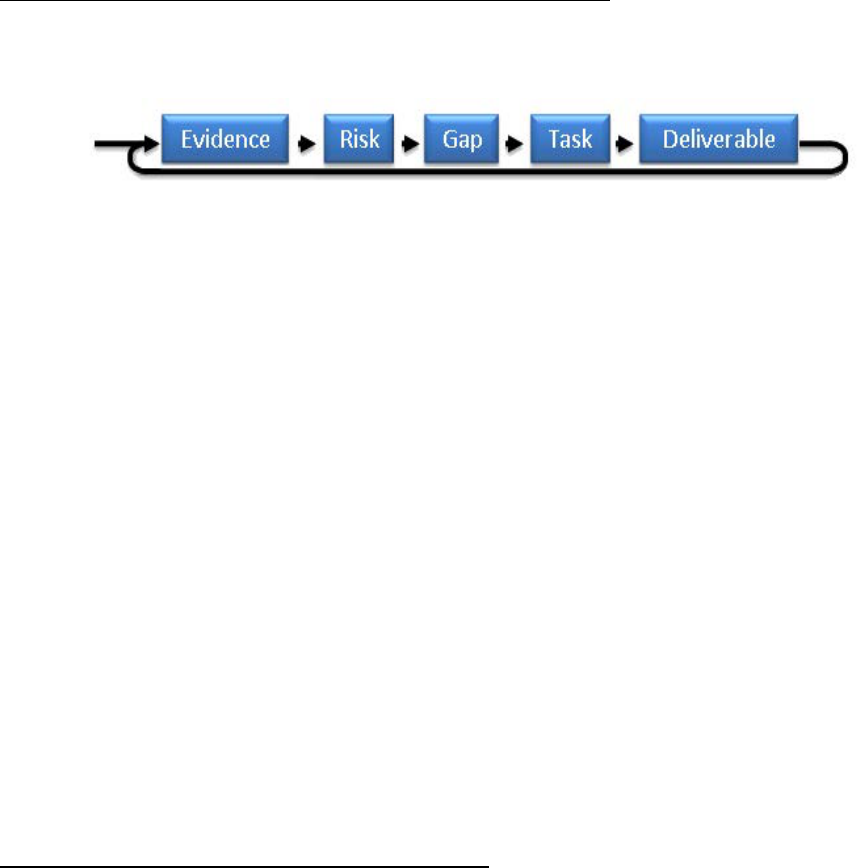
As shown in Figure 1, the development of HRP content has been formulated around the
management architecture:
Figure 1: HRP Management Architecture
Evidence forms the basis for the existence of a risk to the human system. The individual risk
research plans, which are compiled in the Integrated Research Plan (IRP), contain gaps in
knowledge about characterizing or mitigating the risk, and the tasks to be carried out in order to
produce the deliverables needed to fill the gaps and reduce the risk. HRP deliverables are
generally:
1. Knowledge – deliverables that add to the body of knowledge regarding the risk or
concern,
2. Countermeasures – preventive and treatment actions taken to address a risk,
3. Technology Development – hardware and software that enable risk monitoring,
prevention or treatment,
4. Operational Protocols – operational procedures and methods that define a technique or
process for mitigation of the risk, and
5. Guidelines, Requirements, and Standards – information that defines the acceptable levels
of risk.
Information generated by HRP that can inform the status of the risk and anticipated mitigations
are documented in the Risk Summaries in the risk records maintained by the HSRB.
The process for changing HH&P risks is documented in HRP-47069, Human Research Program
Unique Processes, Criteria, and Guidelines (UPCG) document.
Risks and Concerns in the HRP Research Portfolio
The current HRP HH&P risks and concerns and applicable HRP Element assignments are listed
in Tables 3 and 4. Tables 3 and 4 contain the following information:
1. HRP Element: The Element(s) with primary responsibility for the research.
2. Title: Top level wording used to describe the risk or concern.
3. Short Title: An abbreviation of the title which is for HRP use only.

HRP-47052
8
4. (Short Title) Link to HRR: Specific weblinks to the IRP Human Research Roadmap
(HRR) risk breakdown.
These risks and concerns reflect the current risk information presented at the HSRB level. At
this time, there are 23 risks and 2 concerns approved at the HSRB for which research is to be
performed by HRP. Each Element also has the ability to manage the research to a lower fidelity
of risk topic breakdown through their respective research plans (see the HRR,
http://humanresearchroadmap.nasa.gov/ and the links provided in the tables below). The current
disposition status of these risks is captured in Appendix C.
Table 3 – Human System Health and Performance Risks Addressed by HRP
HRP
Element
Title
1
HHC
Risk of Orthostatic Intolerance During Re-Exposure to Gravity (Short Title: OI) OI
Link to HRR
2
HHC
Risk of Injury and Compromised Performance Due to EVA Operations (Short Title:
EVA) EVA Link to HRR
3
HHC
Risk of Impaired Performance Due to Reduced Muscle Mass, Strength and Endurance
(Short Title: Muscle) Muscle Link to HRR
4
HHC
Risk of Cardiac Rhythm Problems (Short Title: Arrhythmia) Arrhythmia Link to HRR
5
HHC
Risk of Reduced Physical Performance Capabilities Due to Reduced Aerobic Capacity
(Short Title: Aerobic) Aerobic Link to HRR
6
HHC
Risk of Adverse Health Event Due to Altered Immune Response (Short Title: Immune)
Immune Link to HRR
7
HHC
Risk of Impaired Control of Spacecraft/Associated Systems and Decreased Mobility
Due to Vestibular/Sensorimotor Alterations Associated with Space Flight (Short Title:
Sensorimotor) Sensorimotor Link to HRR
8
HHC
Risk of Spaceflight-Induced Intracranial Hypertension/Vision Alterations (Short Title:
VIIP) VIIP Link to HRR
9
HHC
Risk of Decompression Sickness (Short Title: DCS) DCS Link to HRR
10
HHC
Risk of Reduced Crew Health and Performance Due to Hypobaric Hypoxia (Short
Title: Exploration Atmosphere) Exploration Atmosphere Link to HRR (To be added
once developed)
11
HHC
Risk of Performance Decrement and Crew Illness Due to Inadequate Food and
Nutrition (Short Title: Food & Nutrition) Food Link to HRR, Nutrition Link to HRR
12
HHC
Risk of Adverse Health Effects Due to Host-Microorganism Interactions (Short Title:
Microhost) Microhost Link to HRR
13
ExMC
Risk of Adverse Health and Performance Effects of Celestial Dust Exposure (Short
Title: Dust) Dust Link to HRR
14
ExMC
Risk of Adverse Health Outcomes and Decrements in Performance Due to In-flight
Medical Conditions (Short Title: ExMC) ExMC Link to HRR
15
ExMC
Risk of Renal Stone Formation (Short Title: Renal) Renal Link to HRR
16
ExMC
&
HHC
Risk of Bone Fracture Due to Spaceflight Induced Changes to Bone (Short Title:
Fracture) Fracture Link to HRR, Osteo Link to HRR
17
ExMC
Risk of Ineffective or Toxic Medications Due to Long Term Storage (Short Title:
Stability) Stability Link to HRR
18
HFBP
Risk of Reduced Crew Performance and of Injury Due to Inadequate Human-System
Interaction Design (Short Title: HSID) Hab Link to HRR, MPTask Link to HRR,
HARI Link to HRR, HCI Link to HRR, Train Link to HRR

HRP-47052
9
HRP
Element
Title
19
HFBP
Risk of Injury from Dynamic Loads (Short Title: Occupant Protection) Occupant
Protection Link to HRR
20
HFBP
Risk of Adverse Cognitive or Behavioral Conditions and Psychiatric Disorders (Short
Title: BMed) BMed Link to HRR
21
HFBP
Risk of Performance Decrements and Adverse Health Outcomes Resulting from Sleep
Loss, Circadian Desynchronization, and Work Overload (Short Title: Sleep) Sleep
Link to HRR
22
HFBP
Risk of Performance and Behavioral Health Decrements Due to Inadequate
Cooperation, Coordination, Communication, and Psychosocial Adaptation within a
Team (Short Title: Team) Team Link to HRR
23
SR
Risk of Adverse Health Outcomes and Performance Decrements Resulting from Space
Radiation Exposure (Short Title: Radiation) CNS Link to HRR, ARS Link to HRR,
Degen Link to HRR, Cancer Link to HRR
Table 4 – Human System Health and Performance Concerns Addressed by HRP
HRP
Element
Title
1
HHC
Concern of Clinically Relevant Unpredicted Effects of Medication (Short Title:
PK/PD) PK/PD Link to HRR
2
HHC
Concern of Intervertebral Disc Damage Upon and Immediately After Re-Exposure to
Gravity (Short Title: IVD) IVD Link to HRR
5.1 RISK ASSESSMENT METHODS
The HRP shall use qualitative and quantitative methods to assess the HH&P risks associated with
human spaceflight for exploration missions.
Rationale: In many cases, there is a large uncertainty associated with the risk due
to lack of controlled spaceflight (or ground analog) experimental evidence. This
HRP requirement is to determine the likelihood and consequences of the risks
using, at minimum, qualitative means, and implementing quantitative methods
when data are available to support a meaningful assessment. The uncertainties
associated with these quantities should be narrowed to the target values identified
by each standard or to the greatest extent practical to facilitate risk mitigation
through proper decisions for exploration hardware and software design and
mission design. The risk assessment information produced by HRP is captured in
the HSRB Risk Summaries.
HRP-47052
10
5.1.1 The HFBP shall qualitatively or quantitatively assess the HFBP-applicable risks
identified in Table 3.
5.1.2 The ExMC shall qualitatively or quantitatively assess the ExMC-applicable risks
identified in Table 3.
5.1.3 The HHC shall qualitatively or quantitatively assess the HHC-applicable risks identified
in Table 3.
5.1.4 The SR shall qualitatively or quantitatively assess the SR-applicable risks identified in
Table 3.
5.2 HRP PROGRAM SCIENCE MANAGEMENT OFFICE (PSMO) SUPPORT
The HRP Program Science Management Office (PSMO) shall develop ways to improve the
approaches for assessing the integration of HH&P risks associated with human spaceflight for
exploration missions.
Rationale: The risks often have inter-relationships and interdependencies. The
PSMO must evaluate the risks to identify and quantify these inter-relationships
and interdependencies, and provide an assessment of the risks from an integrated
perspective. This will help focus HRP efforts and ensure proper decision making.
5.3 COUNTERMEASURE AND TECHNOLOGY DEVELOPMENT
The HRP Elements shall develop countermeasures and technologies, or inform mission and
vehicle requirements, to prevent or mitigate HH&P Risks.
Rationale: Each risk is written with respect to an adverse outcome. The intent of
the HRP is to prevent the adverse outcome from occurring through development
and validation of novel countermeasures (devices, drugs, procedures, etc.),
providing research evidence that supports the mission and vehicle design
processes, and developing design requirements that will mitigate the adverse
outcome. In this context, “mitigate” means “reduce the severity” or “reduce the
probability of the adverse outcome” or both.
HRP-47052
11
5.3.1 The HFBP shall develop countermeasures and technologies, or provide research evidence
to inform mission and vehicle requirements, to prevent or mitigate adverse outcomes of
HFBP-applicable risks identified in Table 3.
5.3.2 The ExMC shall develop countermeasures and technologies, or provide research
evidence to inform mission and vehicle requirements, to prevent or mitigate adverse
outcomes of ExMC-applicable risks identified in Table 3.
5.3.3 The HHC shall develop countermeasures and technologies, or provide research evidence
to inform mission and vehicle requirements, to prevent or mitigate adverse outcomes of
HHC-applicable risks identified in Table 3.
5.3.4 The SR shall develop countermeasures and technologies, or provide research evidence to
inform mission and vehicle requirements, to prevent or mitigate adverse outcomes of SR-
applicable risks identified in Table 3.
5.4 DEVELOPMENT OF METHODS AND TECHNOLOGIES TO MONITOR AND
TREAT
The HRP Elements shall develop methods and technologies to monitor and treat adverse
outcomes of HH&P Risks.
Rationale: If a risk cannot be mitigated adequately, the human must be monitored
for indicators of an adverse outcome, and treatment methods should be developed.
5.4.1 The HFBP shall develop methods and technologies to monitor and treat adverse
outcomes of HFBP-applicable risks identified in Table 3.
5.4.2 The ExMC shall develop methods and technologies to monitor and treat adverse
outcomes of ExMC-applicable risks identified in Table 3.
5.4.3 The HHC shall develop methods and technologies to monitor and treat adverse outcomes
of HHC-applicable risks identified in Table 3.
5.4.4 The SR shall develop methods and technologies to monitor indicators of adverse
outcomes of SR-applicable risks identified in Table 3.
5.5 PROVISION OF SUPPORTING EVIDENCE
The HRP Elements shall provide evidence to support a determination of status for a concern.
Rationale: Elements provide evidence to the HSRB to help determine if a concern
should remain a concern, be recognized as a risk, or be recognized as a topic
which does not require further resources to address.
HRP-47052
12
5.5.1 The HFBP shall provide evidence to support determination of status for HFBP-applicable
concerns identified in Table 4.
5.5.2 The ExMC shall provide evidence to support determination of status for ExMC-
applicable concerns identified in Table 4.
5.5.3 The HHC shall provide evidence to support determination of status for HHC-applicable
concerns identified in Table 4.
5.5.4 The SR shall provide evidence to support determination of status for SR-applicable
concerns identified in Table 4.
6 HRP REQUIREMENTS RELATED TO ENABLING CAPABILITIES
6.1 PROVISION OF ENABLING CAPABILITIES
The HRP shall provide enabling capabilities to facilitate human space exploration with respect to
the human system.
Rationale: Ensuring human exploration requires some infrastructure or activities
that do not readily fall into a specific research and technology development
category. The requirements below are intended to provide NASA with the
necessary infrastructure or capabilities to implement the research and technology
work required to update, inform, and validate standards and to address the risks
relevant to HH&P.
6.1.1 The ISSMP shall integrate and plan for the execution of HRP research tasks requiring
access to space or flight analog environments.
Rationale: Access to space research platforms (i.e. the ISS and all ISS
visiting vehicles that transport crew and/or cargo to and from the ISS) and
flight analogs is required to study and/or validate many of the items in
sections 4 and 5. The ISSMP serves as the service and interface to
integrate across all HRP Elements, and optimize the research plans
requiring analog access. The ISSMP ensures that data generated are
returned to the investigator.
In the course of research and technology development, each HRP Element may encounter the
need to perform studies in a ground-based space analog environment (e.g., bed-rest facility,
Antarctica). Each Element, with support from ISSMP, is responsible for the selection and/or
validation of the appropriate analogs and the necessary planning, integration, and execution.
Large resource commitments to analog facilities must be reflected in the Element Research Plan
so that the cost-benefit to the HRP is clear.

HRP-47052
13
6.1.2 The PSMO and the NASA medical community shall provide a data integration and
management function to ensure proper handling of and access to HRP data.
Rationale: Access to data is critically important to advancing the state of
knowledge of the human system in space. A data integration and
management function includes the proper archiving of historical research
data (e.g., the Life Sciences Data Archive-LSDA) and organizing medical
and research data to provide proper security levels, allow access by query,
and to provide tools to allow analysis of evidence (e.g., Integrated Medical
Model and the Integrated Medical Evidence Database).
6.2 PRESERVATION AND MAINTENANCE OF CORE TECHNICAL
CAPABILITIES AND EXPERTISE
The HRP shall ensure preservation and maintenance of core technical capabilities and expertise
in human research and technology development.
Rationale: The core competencies are those which are necessary to maintain and
nurture an understanding of the existing evidence base regarding risks to humans
due to spaceflight. This requirement involves sustaining and maintaining a
dedicated scientific and management workforce, publicly documenting the
evidence and a robust external scientific community to provide stability over the
multi-decadal implementation of the vision for space exploration. The core
competencies are necessary to facilitate the following:
Strategic planning. The identification and prioritization of the risks to the human
system and development and execution of long-range research plans to quantify,
prevent, and mitigate risks, and treat adverse outcomes requires competency of
both the internal and external research communities to ensure proper and focused
direction.
Acquisition development, planning, and execution. Acquisition of research and
technology development requires core expertise within the civil service to ensure
that the U.S. Government remains a “smart buyer” with respect to research and
technology development for the human system.
Operations support for near-real time and real-time operational decisions
involving the human system and environment. Laboratory facilities and the
expertise to run them and interpret results are necessary to support an ongoing
evaluation of the human system response to the space environment and to support
the medical operations function during a mission. This involves utilizing the
internal HRP community as much as possible, and to some extent, facilitating the
participation of the external community where uniquely specialized expertise
must be sought.
The requirement is written at the HRP level and not specifically allocated to the Program
Elements. However, the Program Elements shall provide inputs regarding their core competency
needs and issues. As part of the annual Planning, Programming, Budgeting, and Execution
HRP-47052
14
(PPBE) process, Program Management will review the core technical capabilities of the Program
Elements and adjust where appropriate.
6.3 COMPLIANCE WITH APPLICABLE DOCUMENTS
The HRP Elements shall ensure that their processes and products comply with the NASA Policy
Directives and NASA Procedural Requirements listed in the table of Applicable Documents in
Section 2.1.
Rationale: Table 1 includes the NASA Policy Directives (NPD) and NASA
Procedural Requirements (NPR) specifically referenced by HRP-47051, HRP
Program Plan. This requirement explicitly states which NPR and NPD are
applicable to the HRP and ensures that the requirement is flowed down to the
Program Element level. Identification of specific NPR or NPD applicability falls
upon each individual Element and Project when the Project Plan is defined. The
intent of this requirement is to ensure HRP compliance with these documents
within the normal processes and product development ongoing in the HRP.
6.4 OPTIMIZATION OF METHODS AND TECHNOLOGIES
The HRP Elements shall develop methods and technologies to reduce human systems resource
requirements (mass, volume, power, data, etc.).
Rationale: Methods and technologies that reduce the human systems resource
requirements for mass, volume, power, data, etc. must be developed to reduce the
overall exploration resource requirements. Each HRP research element must
focus the research on producing countermeasures and technologies that fit within
the extremely limited resource envelopes anticipated for the exploration mission.
An example is the reduction in time dedicated to exercise prescriptions. Present
exercise prescriptions present a large burden on the overall mission timeline.
6.4.1 The HHC shall develop methods and technologies to reduce human systems resource
requirements (mass, volume, power, crew time, etc.).
6.4.2 The HFBP shall develop methods and technologies to reduce human systems resource
requirements (mass, volume, power, crew time, etc.).
6.4.3 The SR shall develop methods and technologies to reduce human systems resource
requirements (mass, volume, power, crew time, etc.).
6.4.4 The ExMC shall develop methods and technologies to reduce human systems resource
requirements (mass, volume, power, crew time, etc.).
HRP-47052
15
APPENDIX A: ACRONYMS AND ABBREVIATIONS
AM
Ascent Module
ARS
Acute Radiation Sickness
Bmed
Behavioral Conditions & Psychiatric Disorders
CM
Crew Module, Configuration Management
CNS
Central Nervous System
CR
Change Request
DCS
Decompression Sickness
Degen
Degenerative
DM
Descent Module
DRM
Design Reference Mission
DSH
Deep Space Habitat
e.g.
For Example
EDL
Entry, Descent, and Landing
ESD
Exploration Systems Development
EVA
Extravehicular Activity
ExMC
Exploration Medical Capability
g
Gravity
Hab
Habitat
HARI
Human & Automation/Robotic Integration
HCI
Human-Computer Interaction
HEOMD
Human Exploration and Operations Mission Directorate
HFBP
Human Factors and Behavioral Performance
HH&P
Human Health & Performance
HHC
Human Health Countermeasures
HMTA
Health & Medical Technical Authority
HRP
Human Research Program
HRPCB
Human Research Program Control Board
HRR
Human Research Roadmap
HSRB
Human System Risk Board
iMED
Integrated Medical Evidence Database
IMM
Integrated Medical Model
IRP
Integrated Research Plan
ISS
International Space Station
ISSMP
ISS Medical Projects
IVD
Intervertebral Disc
JSC
Johnson Space Center
L1
First Lagrangian Point
L2
Second Lagrangian Point
LAS
Launch Abort System
LEO
Low Earth Orbit
LSDA
Life Sciences Data Archive
MCC
Mission Control Center
Microhost
Host-Microorganism
MPCV
Multi-Purpose Crew Vehicle
MPTASK
Mission, Process, and Task
HRP-47052
16
N/A
Not Applicable
NASA
National Aeronautics and Space Administration
NEA
Near-Earth Asteroid
NPD
NASA Procedural Directive
NPR
NASA Procedural Requirements
OCHMO
Office of the Chief Health and Medical Office
OI
Orthostatic Intolerance
Osteo
Osteoporosis
PCA
Program Commitment Agreement
PCN
Page Change Notice
PD
Pharmacodynamics
PEL
Permissible Exposure Limit
PK
Pharmacokinetics
PPBE
Planning, Programming, Budgeting, and Execution
PRD
Program Requirements Document
PSMO
Program Science Management Office
Rev.
Revision
R&T
Research and Technology
REM
Robotics and EVA Module
SA
Spacecraft Adaptor
SEV
Space Exploration Vehicle
SL
Suit Lock/Suit Port
SLSD
Space Life Sciences Directorate
SM
Service Module
SR
Space Radiation
STD
Standards
TBD
To Be Determined
TEI
Trans-Earth Insertion
U.S.
United States
UPCG
Unique Processes, Criteria, and Guidelines
VIIP
Visual Impairment/Intracranial Pressure
Vol.
Volume

HRP-47052
17
APPENDIX B: DRM DESCRIPTIONS AND ASSUMPTIONS
The HSRB identifies several DRM categories against which HH&P risks are to be evaluated and
the OCHMO risk posture developed. These DRM categories are shown in Figure 2.
Source: JSC 66705, Human System Risk Management Plan
Figure 2: HSRB DRM Categories
Examples of missions that would fall into the DRM categories:
Low Earth Orbit: ISS6, ISS12, Commercial Suborbital, commercial visits to ISS, future
commercial platforms in LEO
Deep Space Sortie: MPCV test flights, moon fly around or landing, visits to L1/L2, deep space
excursion
Lunar Visit/Habitation: Staying on the surface more than 30 days (less than 30 days would be
similar)
Deep Space Journey/Habitation: L1/L2 habitation, asteroid visit, journey to planets
Planetary Visit/Habitation: Living on a planetary surface, Mars & extended journey to and
from in microgravity
These DRM categories provide a framework to identify key capabilities and important guiding
drivers and assumptions to help HRP focus its research questions on topics relevant to NASA’s
future activities. Although these mission types share similar HH&P challenges, each also
includes specific challenges that depend on the nature of the mission and the mission
development schedule. The HRP research and technology development
plan/schedule/framework is phased to supply appropriate deliverables in time to meet the
challenges of each mission type.
HRP-47052
18
At this time, the current DRM parameters defined in the Human System Risk Management Plan
are still in development. In order to guide HRP in properly developing and maturing operations
concepts that will inform requirements for the design and operation of space vehicles and
habitats, additional parameters are needed.
This section provides additional assumptions pertaining to general aspects of the specific
missions for which HRP is focusing research plan development, and is intended to complement
agency DRM information. This list hopes to define a more useful set of mission guidelines that
HRP can utilize in its research planning and risk assessment. These DRM assumptions provide
the bounding conditions and trade space for defining future spaceflight capabilities and key
performance drivers required to achieve mission objectives. Information regarding the Space
Exploration Vehicle (SEV), Deep Space Habitat (DSH), and Robotics and EVA Module (REM)
were obtained from ESD 10012, Exploration Systems Development (ESD) Concept of
Operations. Information about missions supported by the Commercial Crew Program is from
CCT-DRM-1110, Revision Basic-3, Crew Transportation System Design Reference Missions.

HRP-47052
19
Low Earth Orbit (LEO): ISS 6-Month Mission Assumptions
Crew Size
• 4 crew members (will have interaction with additional 4 ISS crew members that will be
in 6-month rotation cycle)
Mission Duration
• 6 months (including transit)
Gravity Environment
• Microgravity
Radiation Environment
• LEO – Van Allen Belt
Earth Return
• 1 day or less
Role of Ground Support/Mission Control Center (MCC)
• Real-time communication
• Ground personnel support provided real time
o On-board operations (e.g. monitoring/controlling systems during the crew sleep
period, operating or assisting the operation of robotic systems)
o Managing and replanning schedule as necessary
o Training support (e.g., medical evaluations using ultrasound can be performed with
real time support from a flight surgeon)
Resupply and Sample Return
• 1 resupply mission (e.g. for consumables and spare parts and sample return)
Crew Habitation
• Soyuz spacecraft transportation to/from ISS
• Commercial crew carrier sized for a minimum of 4 people
• ISS volume is approximately 388 cubic meters
Crew Timeline/Activities
• Crew sleep, pre/post sleep activities to include galley operations and personal hygiene,
exercise, review/development of crew planned activities/schedule
• Science/payload operations and vehicle system management/maintenance as required
• Interaction with ground control center
• 1-2 EVAs per increment in a nominal mission
Exercise Equipment
• Available equipment that will allow the crew to perform current exercise prescriptions
HRP Constraints/Implied Requirements
• Adequate vehicle or habitat shielding, dosimetry, and operational procedures in place to
prevent exposures above 30-day permissible dose limits
Pre/Post Mission Assumptions
• Some HRP investigations will allow flexibility in their requirements for significant crew
time in the immediate post-flight measurements (R+1 week especially).
• Medical testing conducted during the first week postflight will occur as usual.
• Requirements with minimal crew time commitments (e.g., blood, urine and saliva
collections) may still be performed.

HRP-47052
20
Low Earth Orbit (LEO): ISS 12-Month Mission Assumptions
Crew Size
• 2 crew members (will have interaction with additional 4 ISS crew members that will be
in 6-month rotation cycle)
Mission Duration
• 12 months (including transit)
Gravity Environment
• Microgravity
Radiation Environment
• LEO – Van Allen Belt
Earth Return
• 1 day or less
Role of Ground Support / Mission Control Center (MCC)
• Real-time communication
• Ground personnel support provided real-time
o On-board operations (e.g., monitoring/controlling systems during the crew sleep
period, operating or assisting the operation of robotic systems)
o Managing and replanning schedule as necessary
o Training support (e.g., medical evaluations using ultrasound can be performed with
real time support from a flight surgeon)
Resupply and Sample Return
• 1-2 resupply missions (e.g., for consumables and spare parts and sample return)
Crew Habitation
• Soyuz spacecraft transportation to/from ISS
• Commercial crew carrier sized for a minimum of 4 people
• ISS volume is approximately 388 cubic meters.
Crew Timeline/Activities
• Crew sleep, pre/post sleep activities to include galley operations and personal hygiene,
exercise, review/development of crew planned activities/schedule.
• Science/payload operations and vehicle system management/maintenance as required
• Interaction with ground control center
• Up to 4 EVAs per crew member in a nominal mission
Exercise Equipment
• Available equipment that will allow the crew to perform current exercise prescriptions.
HRP Constraints/Implied Requirements
• Adequate vehicle or habitat shielding, dosimetry, and operational procedures in place to
prevent exposures above 30-day permissible dose limits.
Pre/Post Mission Assumptions
• Some HRP investigations will allow flexibility in their requirements for significant crew
time in the immediate post-flight measurements (R+1 week especially).
• Medical testing conducted during the first week postflight will occur as usual.
• Requirements with minimal crew time commitments (e.g., blood, urine and saliva
collections) may still be performed.

HRP-47052
21
Deep Space Sortie Mission Assumptions
Crew Size
• 4 crew members
Mission Duration
• 1 month (including transit)
Gravity Environment
• Microgravity
Radiation Environment
• LEO – Van Allen Belt
Earth Return
• Less than 5 days
Role of Ground Support/MCC
• Communication delay of about 2.5 seconds round trip
• Ground personnel support provided in near real-time
o On-board operations (e.g., monitoring/controlling systems during the crew sleep
period)
o Managing and replanning schedule as necessary
o Training support (e.g., medical evaluations using ultrasound can be performed with
near real-time support from a flight surgeon)
• No ground support in terms of ‘watching over the shoulder’ during robotics operations
Crew Habitation
• MPCV:
o Consists of a Crew Module (CM), a Service Module (SM), Spacecraft Adaptor (SA)
and a Launch Abort System (LAS). The CM provides a habitable pressurized volume
to support crew members and cargo during all phases of a given mission.
o Includes flight suits designed for the different roles required to allow crew members
to perform launch, entry, exploration and extravehicular servicing and repair
operations.
• Lunar Lander:
o Consists of a three-module vehicle configuration - the Descent Module (DM), the
Ascent Module (AM), and a Suit Lock/Suit Port (SL) module. The suit ports
minimize the time required for the crew members to don suits and begin surface
EVA, and minimize atmosphere losses.
• EVA Deep Space Suit:
o Allows crew members to perform exploration and extravehicular servicing and repair
operations. The suit provides life support, environmental protection and
communications capability to the EVA crew member while allowing sufficient
mobility to perform dexterous EVA tasks.
o The Block 1 EVA Deep Space Suit is a separate suit element from the MPCV flight
suits and is configured for microgravity EVA capability, designed for upper body
mobility and worksite stabilization. The Block 2 EVA Surface Suit provides surface
EVA capability on objects with a gravity field and no atmosphere, such as the Moon.
• Space Exploration Vehicle (SEV):
o Combines a pressurized cabin and crew member support equipment, a
propulsion/consumables unit, and robotic support packages.
HRP-47052
22
o Provides habitation during transit, serves as an excursion spacecraft at mission
destinations, provides a robotic and robot-assisted exploration capability, and
provides simultaneous EVA capability for two crew members.
o Includes a system for physically securing the vehicle to external objects (e.g. NEAs,
satellites), dexterous manipulators for scientific and servicing/repair operations, and
an astronaut positioning system to facilitate EVA operations.
Sample Return
• All monitoring for microbial or toxic hazards must be performed on board.
• Sample return capability may be available for a very limited amount driven by MPCV or
Lunar Lander vehicle stowage capability.
Crew Timeline/Activities
• Transit:
o Crew sleep, pre/post sleep activities to include galley operations and personal
hygiene, exercise, review/development of crew planned activities/schedule.
o Science/payload operations (dependent on upmass capabilities) and vehicle system
management/maintenance as required
o Interaction with ground control center
o No planned or contingency EVAs during transit time
• Surface Operations:
o TBD EVAs per crew member
o EVA crew members egressing from the vehicle through an airlock or suitport
provided capability
o Paired EVAs that maximize the scientific and operational value of the mission.
Communication Delays
• Around 2.5 seconds round trip while on the lunar surface
Crew Logistics/Food
• No mission resupply to replenish the crew with logistical requirements during the entire
mission. All consumables and spare parts must be available from the habitable volume.
• MPCV and Lunar Lander module will have a food galley with the required capabilities
for the crew to prepare their meals. Majority of the food storage will be contained in the
Lunar Lander module under the required food storage constraints.
Exercise Equipment
• Available equipment that will allow the crew to perform current exercise prescriptions.
HRP Constraints/Implied Requirements
• Adequate vehicle or habitat shielding, dosimetry, and operational procedures in place to
prevent exposures above 30-day permissible dose limits.
Pre/Post Mission Assumptions
• TBD post-flight Baseline Data Collection will still be required, similar to ISS post-flight
protocols.

HRP-47052
23
Lunar Visit/Habitation Mission Assumptions
Crew Size
• 4 crew members
Mission Duration
• 1 year (including transit)
Gravity Environment
• 1/6
th
Earth gravity
Radiation Environment
• Lunar
Earth Return
• 5 days
Role of Ground Support / MCC
• Communication delay of about 2.5 seconds round trip
• Ground personnel support provided in near real-time
o On-board operations (e.g. monitoring/controlling systems during the crew sleep
period)
o Managing and replanning schedule as necessary
o Training (e.g., medical evaluations using ultrasound can be performed with near real-
time support from a flight surgeon)
• No ground support in terms of ‘watching over the shoulder’ during robotics operations
Crew Habitation
• MPCV:
o Consists of a Crew Module (CM), a Service Module (SM), Spacecraft Adaptor (SA)
and a Launch Abort System (LAS). The CM provides a habitable pressurized volume
to support crew members and cargo during all phases of a given mission.
o Includes flight suits designed for the different roles required to allow crew members
to perform launch, entry, exploration and extravehicular servicing and repair
operations.
• Lunar Lander:
o Consists of a three-module vehicle configuration - the Descent Module (DM), the
Ascent Module (AM), and a Suit Lock/Suit Port (SL) module. The suit ports
minimize the time required for the crew members to don suits and begin surface
EVA, and minimize atmosphere losses.
• EVA Deep Space Suit:
o Allows crew members to perform exploration and extravehicular servicing and repair
operations. The suit provides life support, environmental protection and
communications capability to the EVA crew member while allowing sufficient
mobility to perform dexterous EVA tasks.
o The Block 1 EVA Deep Space Suit is a separate suit element from the MPCV flight
suits and is configured for microgravity EVA capability, designed for upper body
mobility and worksite stabilization. The Block 2 EVA Surface Suit provides surface
EVA capability on objects with a gravity field and no atmosphere, such as the Moon.
Robotics
• Robotics and EVA Module (REM):
HRP-47052
24
o Provides infrastructure necessary for extravehicular human and robotic operations,
transportation element maintenance and repair, movement of equipment and
payloads, anchoring or tethering of external bodies (e.g. satellite), extraction of cargo,
and deployment of payloads in Earth orbit for independent entry.
o Consists of a suitlock with two suitports, at least one robotic arm with a grapple
fixture and EVA positioning end effectors, an international docking system standard
interface, an external equipment pallet, a crew lock, and mounting points to which
elements and payload hardware can be mounted.
Sample Return
• All monitoring for microbial or toxic hazards must be performed on board.
• Sample return capability may be available for a very limited amount driven by MPCV or
Lunar Lander vehicle stowage capability.
Crew Timeline/Activities
• Transit:
o Crew sleep, pre/post sleep activities to include galley operations and personal
hygiene, exercise, review/development of crew planned activities/schedule.
o Science/payload operations (dependent on upmass capabilities) and vehicle system
management/maintenance as required
o Interaction with ground control center
o No planned or contingency EVAs during transit time
• Surface Operations:
o TBD EVAs per crew member
o EVA crew members egressing from the vehicle through an airlock or suitport
provided capability
o Paired EVAs that maximize the scientific and operational value of the mission.
Communication Delays
• Around 2.5 seconds round trip while on the lunar surface
Crew Logistics/Food
• No mission resupply to replenish the crew with logistical requirements during the entire
mission. All consumables and spare parts must be available from the habitable volume.
• MPCV and Lunar Lander module will have a food galley with the required capabilities
for the crew to prepare their meals. Majority of the food storage will be contained in the
Lunar Lander module under the required food storage constraints.
Exercise Equipment
• Available equipment that will allow the crew to perform current exercise prescriptions.
HRP Constraints/Implied Requirements
• Adequate vehicle or habitat shielding, dosimetry, and operational procedures in place to
prevent exposures above 30-day permissible dose limits.
• It is assumed that the Mars DRM will follow Level of Care Five standards in NASA-
STD-3001 Vol. 1 for crewmember training and caliber: "The training and caliber of the
caregiver shall be at the physician level, due to the exclusively autonomous nature of the
mission."
Pre/Post Mission Assumptions
• TBD post-flight Baseline Data Collection will still be required, similar to ISS post-flight
protocols.

HRP-47052
25
Deep Space Journey/Habitation Mission Assumptions
Crew Size
• 3 crew members
Mission Duration
• 1 year (including transit)
Gravity Environment
• Microgravity
Radiation Environment
• Deep space
Earth Return
• Weeks to months
Role of Ground Support / MCC
• Communication delay of up to 30 seconds
• Ground personnel support provided in overall ‘batch mode’ rather than immediate or real
time
o On-board operations (e.g., monitoring/controlling systems during crew sleep with
some delay, some delay in supporting operation of robotics systems)
o Managing and replanning schedule with some delay
o Training (e.g., training materials sent in ‘batch mode’, some delay in support from a
flight surgeon for medical evaluations such as ultrasound)
Crew Habitation
• MPCV:
o Consists of a Crew Module (CM), a Service Module (SM), Spacecraft Adaptor (SA)
and a Launch Abort System (LAS). The CM provides a habitable pressurized volume
to support crew members and cargo during all phases of a given mission.
o Includes flight suits designed for the different roles required to allow crew members
to perform launch, entry, exploration and extravehicular servicing and repair
operations.
• Space Exploration Vehicle (SEV):
o Combines a pressurized cabin and crew member support equipment, a
propulsion/consumables unit, and robotic support packages.
o Provides habitation during transit, serves as an excursion spacecraft at mission
destinations, provides a robotic and robot-assisted exploration capability, and
provides simultaneous EVA capability for two crew members.
o Includes a system for physically securing the vehicle to external objects (e.g. NEAs,
satellites), dexterous manipulators for scientific and servicing/repair operations, and
an astronaut positioning system to facilitate EVA operations.
• Deep Space Habitat (DSH):
o Provides a pressurized environment in which crew members live and work during
extended transit phases and while at exploration destinations for longer duration
missions.
o Provides all of the resources necessary to support the crew members during this
timeframe and carries additional supplies and spares for the rest of the stack. The
DSH can be divided into separable pressurized volumes, with each section having at
HRP-47052
26
least one docking port capable of supporting crew members transfer and
accommodating either the MPCV and/or SEV vehicles.
o During integrated stack operations, the DSH provides life support functions
throughout the docked habitable elements. The DSH provides radiation protection
and is expected to include crew member accommodations such as food preparation,
cleaning equipment, photography equipment, and exercise equipment. Pressurized
logistics and spares are stowed as well.
Robotics
• Robotics and EVA Module (REM):
o Provides infrastructure necessary for extravehicular human and robotic operations,
transportation element maintenance and repair, movement of equipment and
payloads, anchoring or tethering of external bodies (e.g. satellite), extraction of cargo,
and deployment of payloads in Earth orbit for independent entry.
o Consists of a suitlock with two suitports, at least one robotic arm with a grapple
fixture and EVA positioning end effectors, an international docking system standard
interface, an external equipment pallet, a crew lock, and mounting points to which
elements and payload hardware can be mounted.
Sample Return
• All monitoring for microbial or toxic hazards must be performed on board.
• No sample return will be possible.
Crew Timeline/Activities
• Transit:
o Crew sleep, pre/post sleep activities to include galley operations and personal
hygiene, exercise, review/development of crew planned activities/schedule.
o Science/payload operations (dependent on upmass capabilities) and vehicle system
management/maintenance as required
o Interaction with ground control center
o No planned or contingency EVAs during transit time
• Surface Operations:
o TBD EVAs per crew member
o During EVA activities, crew will be augmented with robotic support, and will be able
to perform NEA surface operations only utilizing their robotics capabilities.
o Vehicle design will provide a physical containment area for surface samples to isolate
the crewmembers from any Asteroid surface materials that they may bring back to
Earth.
o Surface operations will subject the EVA crew to a possible microgravity field while
on the surface.
Communication Delays
• Expect communication delays between the crew and the ground control center to increase
from zero during Low Earth Orbit (LEO) to up to approximately 30 seconds at NEA
arrival, with the same duration impact during return to Earth.
• Due to the communication delay, the crew is expected to perform autonomous operations
as required.
Crew Logistics/Food
HRP-47052
27
• No mission resupply to replenish the crew of logistical requirements during the entire
mission. All consumables and spare parts must be provided at the start of the mission
and available from the habitable volume.
• Habitation module will have a food galley with the required capabilities for the crew to
prepare their meals. Food storage will be contained in the habitation module under the
required food storage constraints.
Exercise Equipment
• Available equipment that will allow the crew to perform current exercise prescriptions.
HRP Constraints/Implied Requirements
• Adequate vehicle or habitat shielding, dosimetry, and operational procedures in place to
prevent exposures above 30-day permissible dose limits.
• It is assumed that the Mars DRM will follow Level of Care Five standards in NASA-
STD-3001 Vol. 1 for crewmember training and caliber: "The training and caliber of the
caregiver shall be at the physician level, due to the exclusively autonomous nature of the
mission."
Pre/Post Mission Assumptions
• TBD post-flight Baseline Data Collection will still be required, but protocols will need to
consider degree of crew de-conditioning after a 1-yr mission.

HRP-47052
28
Planetary Visit/Habitation Mission Assumptions
Crew Size
• 6 crew members
Mission Duration
• Total mission duration from launch to crew return approximately 3 years
• Transfer to and from Mars on the order of 6 months, the stay on the Martian surface on
the order of 18 months
Gravity Environment
• 3/8
th
Earth Gravity and microgravity
Radiation Environment
• Planetary (i.e., planet has no magnetic poles, limited atmosphere)
Earth Return
• Months
Role of Ground Support / MCC
• Communication delay of up to 22 minutes to Mars surface.
• Ground personnel support provided in overall ‘batch mode’ rather than immediate or
real-time.
o On-board operations (e.g. monitoring/controlling systems during crew sleep with
significant delay - crew must be able to stabilize systems for all contingencies for up
to 44 minutes without any ground assistance, significant delay in supporting operation
of robotic systems - autonomous operations during the communication delay)
o Managing and replanning schedule with significant delay
o Training (e.g., training materials sent in ‘batch mode’, significant delay in support
from a flight surgeon for medical evaluations such as ultrasound)
Crew Habitation
• MPCV:
o Consists of a Crew Module (CM), a Service Module (SM), Spacecraft Adaptor (SA)
and a Launch Abort System (LAS). The CM provides a habitable pressurized volume
to support crew members and cargo during all phases of a given mission.
o Includes flight suits designed for the different roles required to allow crew members
to perform launch, entry, exploration and extravehicular servicing and repair
operations.
• Deep Space Habitat (DSH):
o Provides a pressurized environment in which crew members live and work during
extended transit phases and while at exploration destinations for longer duration
missions.
o Provides all of the resources necessary to support the crew members during this
timeframe and carries additional supplies and spares for the rest of the stack. The
DSH can be divided into separable pressurized volumes, with each section having at
least one docking port capable of supporting crew members transfer and
accommodating either the MPCV and/or SEV vehicles.
o During integrated stack operations, the DSH provides life support functions
throughout the docked habitable elements. The DSH provides radiation protection
and is expected to include crew member accommodations such as food preparation,
HRP-47052
29
cleaning equipment, photography equipment, and exercise equipment. Pressurized
logistics and spares are stowed as well.
• Surface Habitat:
o (Details to be added once available.)
Crew Timeline/Activities
• Transit:
o Crew sleep, pre/post sleep activities to include galley operations and personal
hygiene, exercise, review/development of crew planned activities/schedule.
o Science/payload operations (dependent on upmass capabilities) and vehicle system
management/maintenance as required
o Interaction with ground control center
o No planned or contingency EVAs during transit
• Surface Operations:
o General outline of crew activities established before the launch, but updated
throughout the mission. Outline contains detailed activities to ensure initial crew
safety, basic assumptions for initial science activities, schedule of periodic vehicle
and system checkout, and plan for certain number of sorties. The crew will plan
specific activities derived from the general objectives defined on Earth.
o Landing operations expected to be fully automated with minimal crew interaction
during the landing sequence.
o Crew will be in a recumbent position during all Entry, Descent, and Landing (EDL)
operations.
o After crew acclimation period of a few weeks, initial surface activities would focus
on transitioning from a “Lander mode” to a fully functional surface habitat.
o During the 18-month stay on the Martian Surface, the six (6) crewmembers are
expected to perform multiple EVAs and interact as directly as possible with the
planet. Field work to be completed in the vicinity of the surface base via EVAs
assisted by pressurized and unpressurized rovers.
− Mars surface EVAs would be conducted by a minimum of two people and
maximum of four.
− If unpressurized rovers are used, an additional operational constraint would be
imposed on the EVA team.
− If one rover is used, the EVA team would be constrained to operate within
rescue range of the surface base.
Communication Delays/Dropouts
• Expected communication delays between the crew and the ground control center will
increase from zero during LEO to up to 6-8 minutes at Mars arrival with the same
duration impact during return to Earth. During the Mars surface operations, these delays
could go up to 22 minutes.
• Due to the communication delay, the crew is expected to perform autonomous operations
as required.
• Due to communication delays and periods of unavailable communication, the crew is
expected to perform autonomous operations as required.
Crew Logistics/Food
• The mission to Mars will consist of the crew habitation modules listed above (MPCV,
DSH) and surface habitat.
HRP-47052
30
• All consumables and spare parts must be provided at the start of the mission and
available from the habitable volume.
• Food carried aboard the transit habitat includes transit consumables needed for the round-
trip journey plus contingency consumables required to maintain the crew should all or
part of the surface mission be aborted.
• If the crew is forced to return to the orbiting vehicle, which would be used as an orbital
“safe haven” until the Trans-Earth Insertion (TEI) window opens, any contingency food
remaining onboard the crewed vehicle would be jettisoned prior to the TEI burn to return
home.
• The habitation module will have a food galley with the required capabilities for the crew
to prepare their meals. Food storage will be contained in the cargo module under the
required food storage constraints.
Resupply and Sample Return
• No mission resupply to replenish the crew of logistical requirements.
• All monitoring for microbial or toxic hazards must be performed on board.
• Sample return feasibility still to be determined.
Exercise Equipment
• Available equipment that will allow the crew to perform current exercise prescriptions.
HRP Constraints/Implied Requirements
• During Mars atmosphere entry (5-g), crew will be in a recumbent position until landing
operations are complete. The vehicle design will not require the crew to be in an upright
standing posture during entry.
• Countermeasures that support the Orthostatic Intolerance (OI) will be provided in support
to any OI related events (e.g., Mars atmosphere entry).
• Adequate vehicle or habitat shielding, dosimetry, and operational procedures in place to
prevent exposures above 30-day permissible dose limits.
• It is assumed that the Mars DRM will follow Level of Care Five standards in NASA-
STD-3001 Vol. 1 for crewmember training and caliber: "The training and caliber of the
caregiver shall be at the physician level, due to the exclusively autonomous nature of the
mission."
Pre/Post Mission Assumptions
• TBD post-flight Baseline Data Collection will still be required, but protocols will need to
consider degree of crew de-conditioning after a 3-yr mission.
HRP-47052
31
APPENDIX C: HUMAN RISK DISPOSITIONS FOR ALL DRMS
Each risk is to be reviewed annually by the HSRB and a determination made of the risk posture
for each DRM category. This posture is reached after discussion about the likelihood and
consequence mapping on the LxC tables of the Risk Summaries and is communicated via the
Risk Disposition and Risk Disposition Rationale fields. The assessment considers if a risk can
be accepted as is or if further mitigation effort is warranted (see JSC 66705, paragraphs 3.4.1
through 3.4.5).
The following chart summarizes and depicts the risk dispositions for all the human system risks
in the HSRB risk portfolio, a subset of which is worked by HRP. The disposition for each risk is
given by operations or long term health consequence, and by DRM category.

HRP-47052
32
In-Flight Operations
Long-term Health
