
09/19/2024
CS321629-AV
1
2024–2025 COVID-19 Vaccine
Immunization Schedule
for People 6 Months of Age and Older
The following tables provide COVID-19 vaccination schedules based on age, health status, and product. For detailed
guidance see Interim Clinical Considerations for Use of COVID-19 Vaccines | CDC.
Table 1a. For people who are NOT moderately or severely immunocompromised
*
2024-25 Moderna COVID-19 Vaccine
Vaccine type: mRNA | Do NOT use any previously available Moderna COVID-19 vaccine products.
If current
age is:
And the COVID-19 vaccination history is: Then: Administer:
6 months
through
4 years
†
Unvaccinated (0 doses)
Give a 2-dose initial series.
• Dose 1 now.
• Dose 2 at least 4–8 weeks after Dose 1.
‡
0.25 mL/25 µg
in a manufacturer-filled
syringe (MFS)
Intramuscular (IM) injection
1 previous dose of any Moderna COVID-19
Vaccine (Dose 1)
§
Complete the series. Give:
• Dose 2 at least 4–8 weeks after Dose 1.
‡
2 or more previous doses of any Moderna
COVID-19 vaccine, NOT including at least
1 dose of 2024–25 vaccine
§
Give 1 dose at least 8 weeks after the
last dose.
2 or more previous doses of any Moderna
COVID-19 vaccine, INCLUDING at least 1 dose
of 2024–25 vaccine
§
No further doses are indicated.
5 through
11 years
¶
Unvaccinated (0 doses) Give 1 dose now.
0.25 mL/25 µg
in a manufacturer-filled
syringe (MFS)
Intramuscular (IM) injection
Any number of previous doses of COVID-19
vaccine, NOT including at least 1 dose of
2024–25 vaccine
Give 1 dose at least 8 weeks after the last
dose.
Any number of previous doses of COVID-19
vaccine, INCLUDING at least 1 dose of
2024–25 vaccine
No further doses are indicated.
12 years
and older
Unvaccinated (0 doses) Give 1 dose now.
0.5 mL/50 µg
in a manufacturer-filled
syringe (MFS)
Intramuscular (IM) injection
Any number of previous doses of COVID-19
vaccine, NOT including at least 1 dose of
2024–25 vaccine
Give 1 dose at least 8 weeks after the
last dose.
Any number of previous doses of COVID-19
vaccine, INCLUDING at least 1 dose of
2024–25 vaccine
**
No further doses are indicated.
* People with a recent SARS-CoV-2 infection may consider delaying vaccination by 3 months from symptom onset or positive test (if infection was asymptomatic).
† Children 6 months through 4 years of age should receive the same vaccine product for all doses. In the following situations, a different age-appropriate COVID-19 vaccine product may
be administered: the same vaccine is not available at the time of the clinic visit, the previous dose is unknown, the person would otherwise not receive a recommended dose, or the
person starts but is unable to complete a vaccination series with the same vaccine due to a contraindication.
‡ An 8-week interval between the first and second doses of Moderna COVID-19 Vaccine might be optimal for some people, as it might reduce the rare risk of myocarditis and pericarditis
associated with COVID-19 vaccines.
§ If mRNA vaccine is administered from different manufacturers, a 3-dose initial series should be followed:
•
Children who received Dose 1 from one manufacturer but will receive subsequent dose(s) from a different manufacturer, administer:
•
Dose 2 at least 4–8 weeks after Dose 1.
•
Dose 3 at least 8 weeks after Dose 2.
•
Children who received 2 doses of vaccine from different manufacturers, administer Dose 3 at least 8 weeks after Dose 2.
¶ CDC recommends that people receive the age-appropriate vaccine product and dosage based on their age on the day of vaccination. Children who turn 5 years of age during the initial
series, administer 1 dose at least 4–8 weeks after Dose 1. There is no dosage change. No further doses are indicated.
** If the immunization history is only 1 dose of 2024–25 Novavax COVID-19 vaccine, see Table 1c for detailed guidance.
09/19/2024
CS321629-AV
2
2024–2025 COVID-19 Vaccine
Immunization Schedule
for People 6 Months of Age and Older
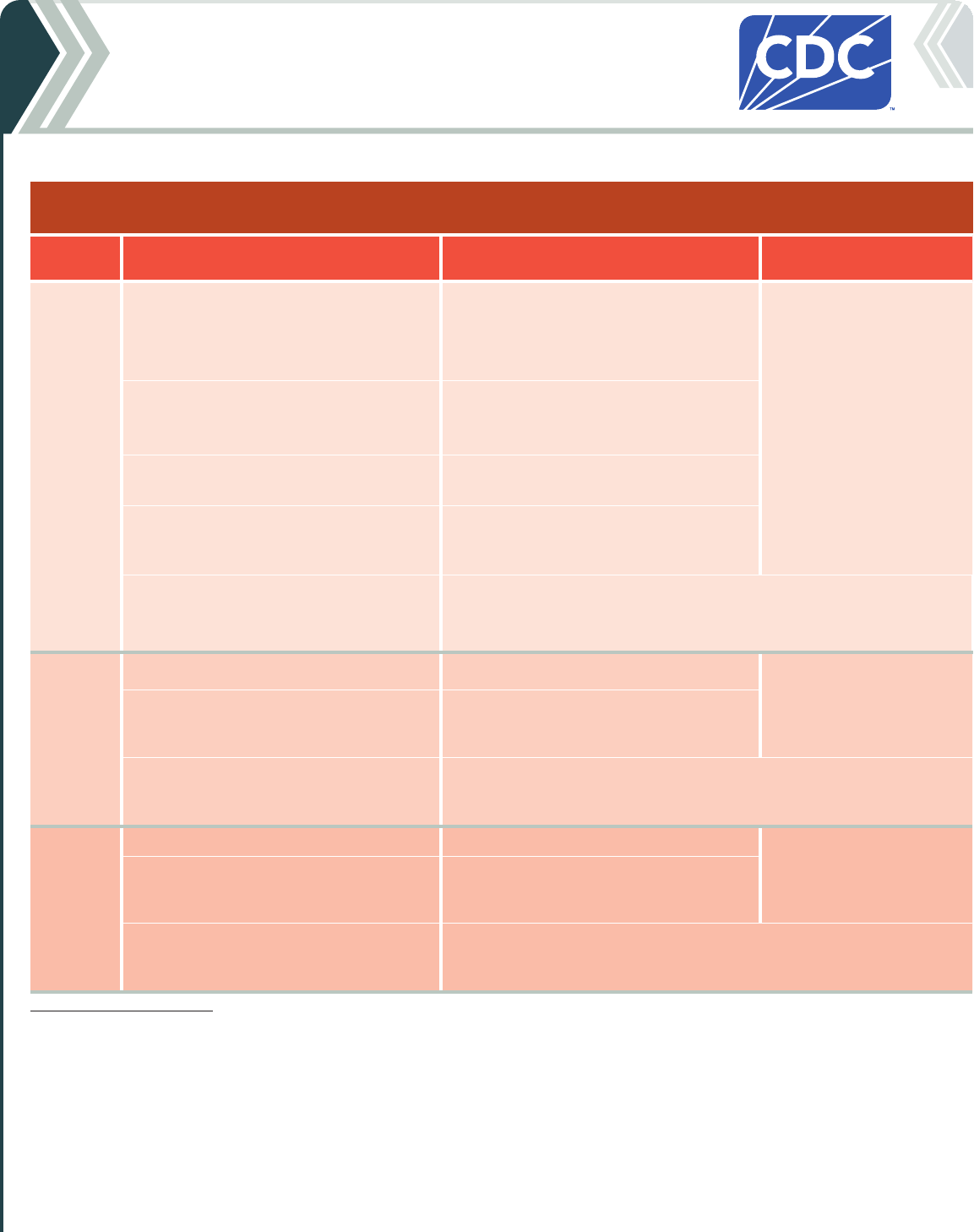
Table 1b. For people who are NOT moderately or severely immunocompromised
*
2024-25 Pfizer-BioNTech COVID-19 Vaccine
Vaccine type: mRNA | Do NOT use any previously available Pfizer-BioNTech COVID-19 vaccine products.
If current
age is:
And the COVID-19 vaccination history is: Then: Administer:
6 months
through
4 years
†
Unvaccinated (0 doses)
Give a 3-dose initial series.
• Dose 1 now.
• Dose 2 at least 3-8 weeks after Dose 1.
‡
• Dose 3 at least 8 weeks after Dose 2.
0.3 mL/3 µg from
a yellow-capped
multidose vial
Intramuscular (IM) injection
1 previous dose of any Pfizer-BioNTech
COVID-19 Vaccine (Dose 1)
§
Complete the before series. Give:
• Dose 2 at least 3–8 weeks after Dose 1.
‡
• Dose 3 at least 8 weeks after Dose 2.
2 previous doses of any Pfizer-BioNTech
COVID-19 Vaccine (Doses 1 and 2)
§
Complete the series. Give:
• Dose 3 at least 8 weeks after Dose 2.
3 or more previous doses of any Pfizer-
BioNTech COVID-19 vaccine, NOT including
at least 1 dose of 2024–25 COVID-19 vaccine
§
Give 1 dose at least 8 weeks after the
last dose.
3 or more previous doses of any Pfizer-
BioNTech COVID-19 vaccine, INCLUDING at
least 1 dose of 2024–25 COVID-19 vaccine
No further doses are indicated.
5 through
11 years
¶
Unvaccinated (0 doses) Give 1 dose now.
0.3 mL/10 µg from a blue-
capped single dose vial
Intramuscular (IM) injection
Any number of previous doses of COVID-19
vaccine, NOT including at least 1 dose of
2024–25 COVID-19 vaccine
Give 1 dose at least 8 weeks after the
last dose.
Any number of previous doses of COVID-19
vaccine, INCLUDING at least 1 dose of 2024–
25 COVID-19 vaccine
No further doses are indicated.
12 years
and older
Unvaccinated (0 doses) Give 1 dose now.
0.3 mL/30 µg
in a manufacturer-filled
syringe (MFS)
Intramuscular (IM) injection
Any number of previous doses of COVID-19
vaccine, NOT including at least 1 dose of
2024–25 COVID-19 vaccine
Give 1 dose at least 8 weeks after the
last dose.
Any number of previous doses of COVID-19
vaccine, INCLUDING at least 1 dose of
2024–25 COVID-19 vaccine
**
No further doses are indicated.
* People with a recent SARS-CoV-2 infection may consider delaying vaccination by 3 months from symptom onset or positive test (if infection was asymptomatic).
† Children 6 months through 4 years of age should receive the same vaccine product for all doses. In the following situations, a different age-appropriate COVID-19 vaccine product may
be administered: the same vaccine is not available at the time of the clinic visit, the previous dose is unknown, the person would otherwise not receive a recommended dose, or the
person starts but is unable to complete a vaccination series with the same vaccine due to a contraindication.
‡ An 8-week interval between the first and second doses of Pfizer-BioNTech COVID-19 Vaccine might be optimal for some people, as it might reduce the rare risk of myocarditis and
pericarditis associated with COVID-19 vaccines.
§ If mRNA vaccine is administered from different manufacturers, a 3-dose initial series should be followed:
•
Children who received Dose 1 from one manufacturer but will receive subsequent dose(s) from a different manufacturer, administer:
•
Dose 2 at least 4–8 weeks after Dose 1.
•
Dose 3 at least 8 weeks after Dose 2.
•
Children who received 2 doses of vaccine from different manufacturers, administer Dose 3 at least 8 weeks after Dose 2.
¶ CDC recommends that people receive the age-appropriate vaccine product and dosage based on their age on the day of vaccination. Children who turn 5 years of age during the initial
series, administer the age-appropriate dose 0.3 mL/10 µg. If the 10 µg dose is the second dose, administer at least 3–8 weeks after the first dose; if it is the third dose, administer at least
8 weeks after the second dose. No further doses are indicated.
**
If the immunization history is only 1 dose of 2024–25 Novavax COVID-19 vaccine, see Table 1c for detailed guidance.

09/19/2024
CS321629-AV
3
2024–2025 COVID-19 Vaccine
Immunization Schedule
for People 6 Months of Age and Older
Table 1c. For people who are NOT moderately or severely immunocompromised
*
2024-25 Novavax COVID-19 Vaccine
Vaccine type: Protein subunit | Do NOT use any previously available Novavax COVID-19 vaccine products.
If current
age is:
And the COVID-19 vaccination history is: Then: Administer:
12 years
and older
Unvaccinated (0 doses)
Give a 2-dose initial series.
• Dose 1 now
• Dose 2 at least 3–8 weeks after Dose 1
†
0.5 mL/5 µg rS protein
and 50 µg Matrix-M
adjuvant
in a manufacturer-filled
syringe (MFS)
Intramuscular (IM)
injection
1 previous dose of 2024-25 Novavax
COVID-19 Vaccine (Dose 1)
Give Dose 2 at least 3–8 weeks after the
last dose.
†
Any number of previous doses of
COVID-19 vaccine, NOT including at least
1 dose of 2024–25 vaccine
Give 1 dose at least 8 weeks after the last dose
Any number of previous doses of
COVID-19 vaccine, WITH at least 1 dose of
2024–25 vaccine
‡
No further doses are indicated.
* Persons with a recent SARS-CoV-2 infection may consider delaying vaccination by 3 months from symptom onset or positive test (if infection was asymptomatic).
† An 8-week interval between the first and second doses of Novavax COVID-19 vaccine might be optimal for some people, as it might reduce the rare risk of myocarditis and pericarditis
associated with COVID-19 vaccines.
‡ If the immunization history is only 1 dose of 2024–25 Novavax COVID-19 vaccine (Dose 1), administer 1 dose of 2024–25 Novavax vaccine at least 3-8 weeks after Dose 1.
09/19/2024
CS321629-AV
4
2024–2025 COVID-19 Vaccine
Immunization Schedule
for People 6 Months of Age and Older
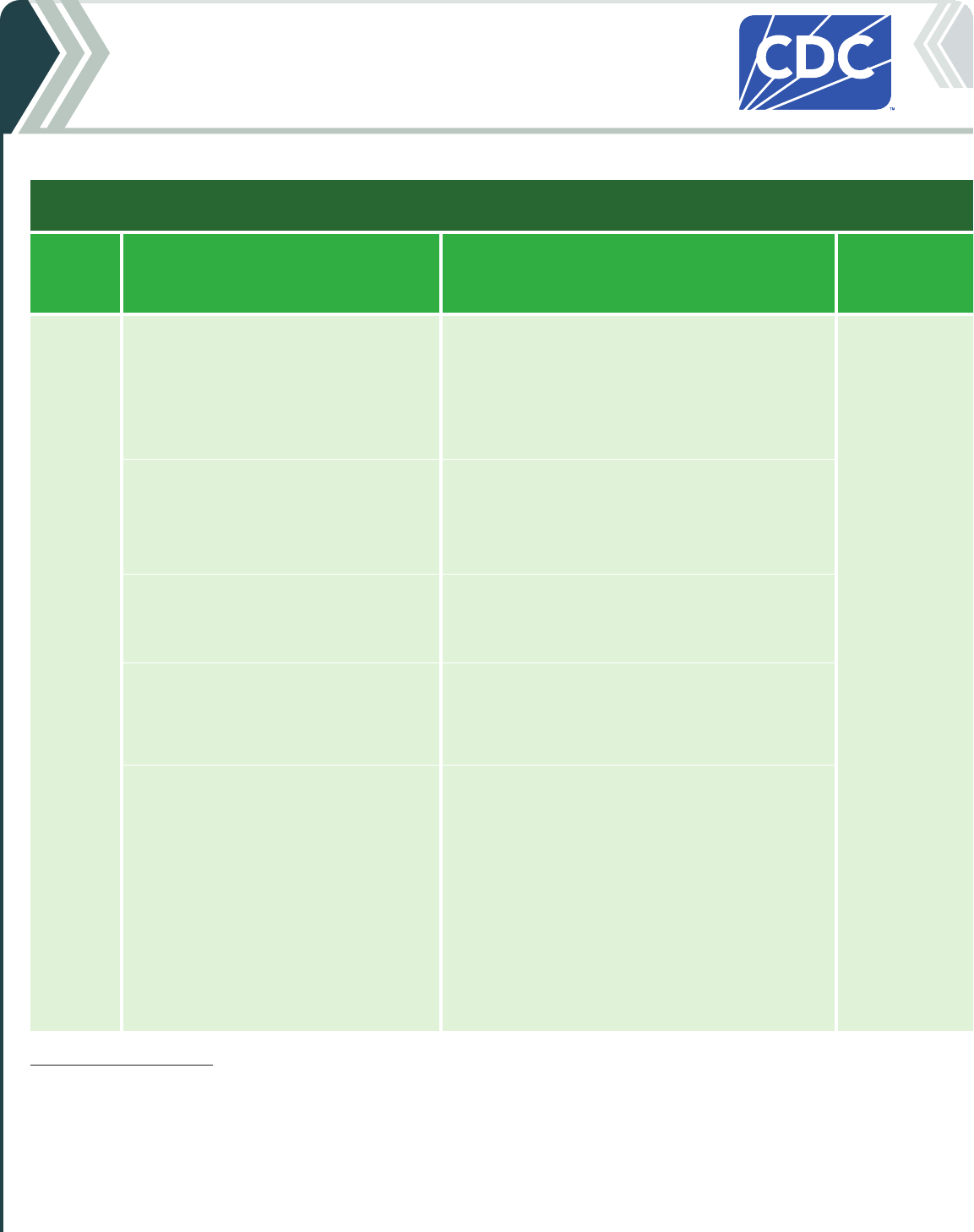
Table 2a. For people who ARE moderately or severely immunocompromised
2024-25 Moderna COVID-19 Vaccine
Vaccine type: mRNA | Do NOT use any previously available Moderna COVID-19 vaccine products.
If current
age is:
And the COVID-19 vaccination history is: Then: Administer:
6 months
through
4 years
*
Unvaccinated (0 doses)
Give a 3-dose initial series.
†
• Dose 1 now.
• Dose 2 at least 4 weeks after Dose 1.
• Dose 3 at least 4 weeks after Dose 2.
0.25 mL/25 µg in
a manufacturer-
filled syringe
(MFS)
Intramuscular
(IM) injection
1 previous dose of any Moderna COVID-19
Vaccine (Dose 1)
‡
Complete the series.
†
Give:
• Dose 2 at least 4 weeks after Dose 1.
• Dose 3 at least 4 weeks after Dose 2.
2 previous doses of any Moderna COVID-19
Vaccine (Doses 1 and 2)
‡
Complete the series.
†
Give:
• Dose 3 at least 4 weeks after Dose 2.
3 or more previous doses of Moderna
COVID-19 Vaccine, NOT including at least 1
dose of 2024–25 COVID-19 vaccine
‡
Give 1 dose at least 8 weeks after the last dose.
3 or more previous doses of Moderna
COVID-19 Vaccine, INCLUDING at least 1
dose of 2024–25 COVID-19 vaccine
• These children may receive 1 additional dose at
least 8 weeks following the last recommended
dose.
• Further additional dose(s) may be administered,
informed by the clinical judgement of a
healthcare provider and personal preference and
circumstances.
• Any further additional doses should be
administered at least 8 weeks after the last
COVID-19 vaccine dose.
* Children 6 months through 4 years of age should receive the same vaccine products for all doses.
† In the following situations, a different age-appropriate COVID-19 vaccine product may be administered: the same vaccine is not available at the time of the clinic visit, the previous
dose is unknown, the person would otherwise not receive a recommended dose, or the person starts but is unable to complete a vaccination series with the same vaccine due to a
contraindication.
‡ If mRNA vaccine is administered from different manufacturers, complete the recommended 3-dose series.
•
For people who received Dose 1 from one manufacturer but will receive subsequent dose(s) from a different manufacturer, administer:
•
Dose 2 at least 4 weeks after Dose 1.
•
Dose 3 at least:
8 weeks after Dose 2 for children 6 months through 4 years of age.
4 weeks after Dose 2 for people 5 years of age and older.
09/19/2024
CS321629-AV
5
2024–2025 COVID-19 Vaccine
Immunization Schedule
for People 6 Months of Age and Older
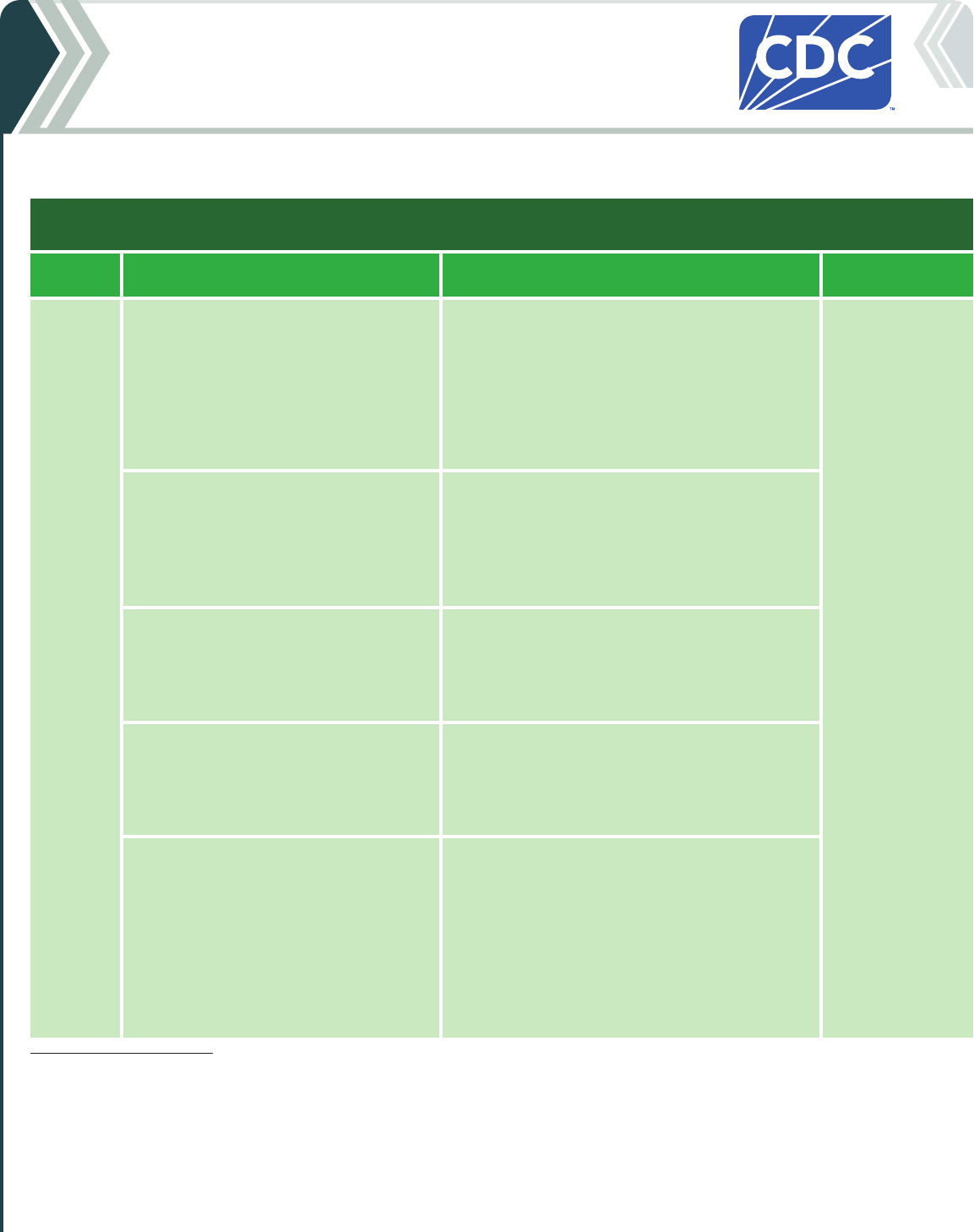
Table 2a. For people who ARE moderately or severely immunocompromised Continued
2024-25 Moderna COVID-19 Vaccine - CONTINUED
Vaccine type: mRNA | Do NOT use any previously available Moderna COVID-19 vaccine products.
If current
age is:
And the COVID-19 vaccination history is: Then: Administer:
5 through
11 years
of age
*
Unvaccinated (0 doses)
Give a 3-dose initial series.
†
• Dose 1 now
• Dose 2 at least 4 weeks after Dose 1.
• Dose 3 at least 4 weeks after Dose 2.
0.25 mL/25 µg in a
manufacturer-filled
syringe (MFS)
Intramuscular (IM)
injection
1 previous dose of any Moderna COVID-19
Vaccine (Dose 1)
‡
Complete the series.
†
Give:
• Dose 2 at least 4 weeks after Dose 1.
• Dose 3 at least 4 weeks after Dose 2.
2 previous doses of any Moderna
COVID-19 Vaccine (Doses 1 and 2)
‡
Complete the series.
†
Give:
• Dose 3 at least 4 weeks after Dose 2.
3 or more doses Moderna COVID-19
Vaccine, NOT including at least 1 dose of
2024–25 vaccine
‡
Give 1 dose at least 8 weeks after the last dose.
3 or more doses Moderna COVID-19
Vaccine, INCLUDING at least 1 dose of
2024–25 vaccine
1 additional dose may be administered at least 8
weeks following the last dose.
Further additional dose(s) may be administered,
informed by the clinical judgement of a health
care provider and personal preference and
circumstances. Administer additional doses at least
8 weeks after the last dose.
* People 5 years of age and older should receive the same vaccine product for the recommended 3-dose series. Additional doses may be any age-appropriate product.
† In the following situations, a different age-appropriate COVID-19 vaccine product may be administered: the same vaccine is not available at the time of the clinic visit, the previous
dose is unknown, the person would otherwise not receive a recommended dose, or the person starts but is unable to complete a vaccination series with the same vaccine due to a
contraindication.
‡ If mRNA vaccine is administered from different manufacturers, complete the recommended 3-dose series.
•
For people who received Dose 1 from one manufacturer but will receive subsequent dose(s) from a different manufacturer, administer:
•
Dose 2 at least 4 weeks after Dose 1.
•
Dose 3 at least:
8 weeks after Dose 2 for children 6 months through 4 years of age.
4 weeks after Dose 2 for people 5 years of age and older.

09/19/2024
CS321629-AV
6
2024–2025 COVID-19 Vaccine
Immunization Schedule
for People 6 Months of Age and Older
Table 2a. For people who ARE moderately or severely immunocompromised Continued
2024-25 Moderna COVID-19 Vaccine - CONTINUED
Vaccine type: mRNA | Do NOT use any previously available Moderna COVID-19 vaccine products.
If current
age is:
And the COVID-19 vaccination history is: Then: Administer:
12 years
and
older
*†
Unvaccinated 0 doses
Give a 3-dose initial series.
‡
• Dose 1 now.
• Dose 2 at least 4 weeks after Dose 1.
• Dose 3 at least 4 weeks after Dose 2.
0.5 mL/50 µg
in a
manufacturer-
filled syringe
(MFS)
Intramuscular
(IM) injection
1 previous dose of any Moderna COVID-19
Vaccine (Dose 1)
§
Complete the series.
‡
Give:
• Dose 2 at least 4 weeks after Dose 1.
• Dose 3 at least 4 weeks after Dose 2.
2 previous doses of any Moderna COVID-19
Vaccine (Doses 1 and 2)
§
Complete the series.
‡
Give:
• Dose 3 at least 4 weeks after Dose 2.
3 or more previous doses of any mRNA
COVID-19 Vaccine, NOT including at least
1 dose of 2024–25 COVID-19 vaccine
Give 1 dose at least 8 weeks after the
last dose.
3 or more previous doses of any mRNA
COVID-19 Vaccine, INCLUDING at least 1
dose of 2024–25 COVID-19 vaccine
1 additional dose may be administered at least 8 weeks
following the last dose.
Further additional dose(s) may be administered,
informed by the clinical judgement of a health care
provider and personal preference and circumstances.
Administer additional doses at least 8 weeks after the
last dose.
2 or more doses of Novavax, NOT
including at least 1 dose of 2024–25
COVID-19 vaccine
Give 1 dose at least 8 weeks after the previous dose.
2 or more doses of Novavax, INCLUDING
at least 1 dose of 2024–25 COVID-19
vaccine
• People who are moderately or severely
immunocompromised may receive 1 additional dose
at least 8 weeks following the last recommended
dose.
• Further additional dose(s) may be administered,
informed by the clinical judgement of a health care
provider and personal preference and circumstances.
• Any further additional doses should be administered
at least 8 weeks after the last COVID-19 vaccine dose
* People 5 years of age and older should receive the same vaccine product for the recommended 3-dose series. Additional doses may be any age-appropriate product.
† CDC recommends that people receive the age-appropriate vaccine product and dosage based on their age on the day of vaccination. Children who turn 12 years of age during the
3-dose series should complete the series with age-appropriate dose 0.5 mL/50µg.
‡ In the following situations, a different age-appropriate COVID-19 vaccine product may be administered: the same vaccine is not available at the time of the clinic visit, the previous
dose is unknown, the person would otherwise not receive a recommended dose, or the person starts but is unable to complete a vaccination series with the same vaccine due to a
contraindication.
§ If mRNA vaccine is administered from different manufacturers, complete the recommended 3-dose series.
For people who received Dose 1 from one manufacturer but will receive subsequent dose(s) from a different manufacturer, administer:
•
Dose 2 at least 4 weeks after Dose 1.
•
Dose 3 at least:
•
8 weeks after Dose 2 for children 6 months through 4 years of age.
•
4 weeks after Dose 2 for people 5 years of age and older.
09/19/2024
CS321629-AV
7
2024–2025 COVID-19 Vaccine
Immunization Schedule
for People 6 Months of Age and Older
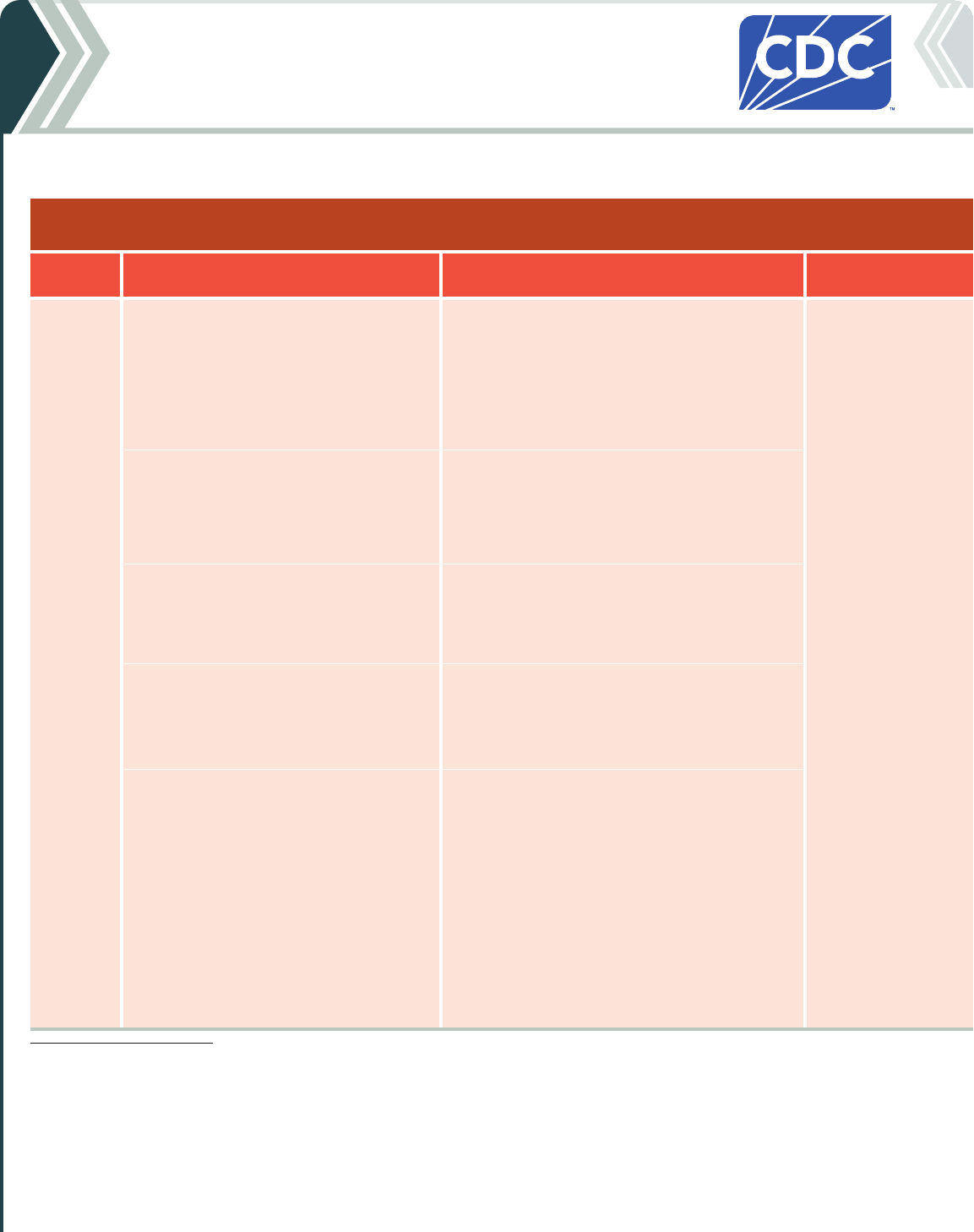
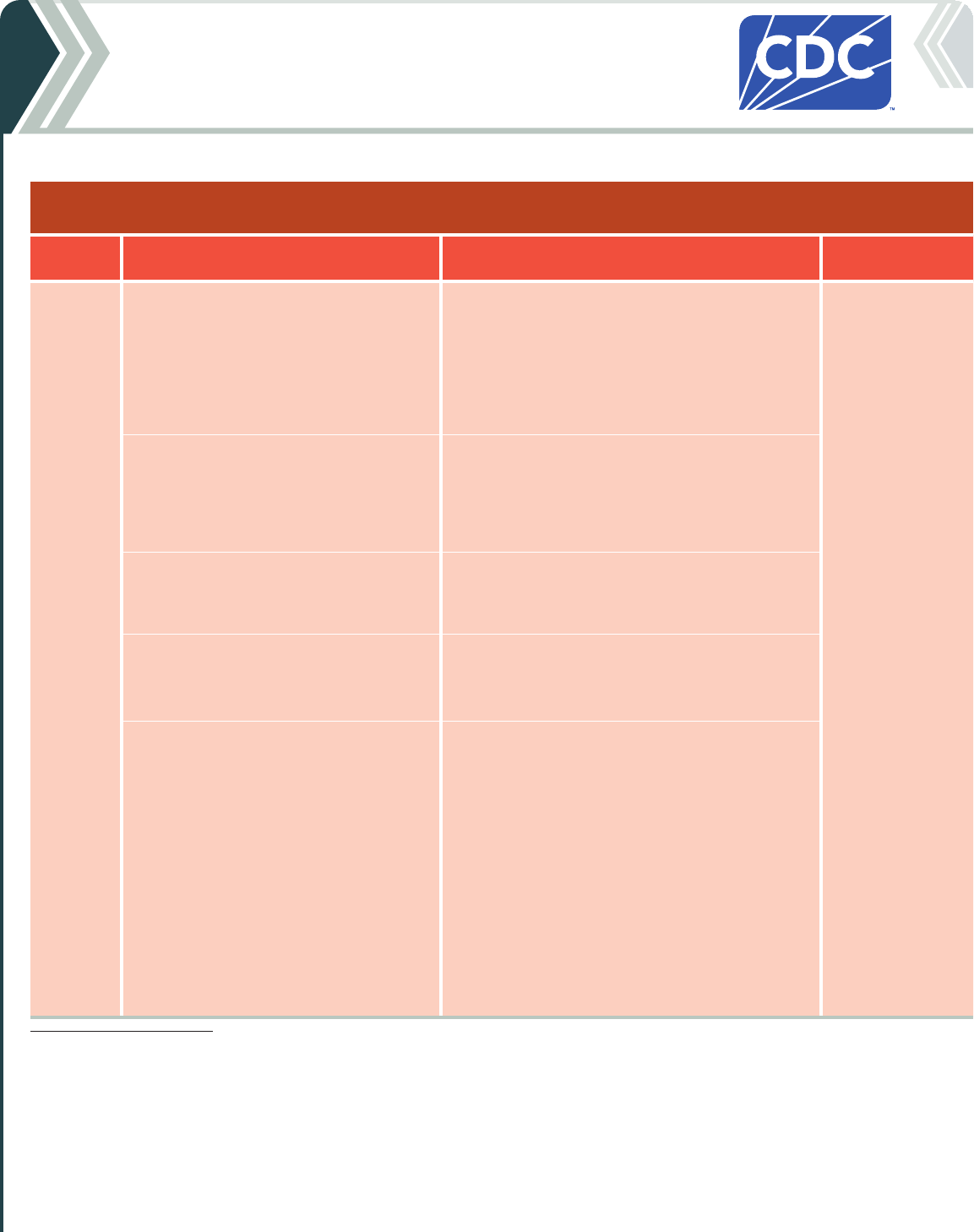
Table 2b. For people who ARE moderately or severely immunocompromised
2024-25 Pfizer-BioNTech COVID-19 Vaccine
Vaccine type: mRNA | Do NOT use any previously available Pfizer-BioNTech COVID-19 vaccine products.
If current
age is:
And the COVID-19 vaccination history is: Then: Administer:
6 months
through
4 years
*
Unvaccinated (0 doses)
Give a 3-dose initial series.
†
• Dose 1 now.
• Dose 2 at least 3 weeks after Dose 1.
• Dose 3 at least 8 weeks after Dose 2.
0.3 mL/3 µg from
a yellow-capped
multidose vial
Intramuscular (IM)
injection
1 previous dose of any Pfizer-BioNTech
COVID-19 Vaccine (Dose 1)
‡
Complete the series.
†
Give:
• Dose 2 at least 3 weeks after Dose 1.
• Dose 3 at least 8 weeks after Dose 2.
2 previous doses of any Pfizer-BioNTech
COVID-19 Vaccine (Doses 1 and 2)
‡
Complete series.
†
Give:
• Dose 3 at least 8 weeks after Dose 2.
3 or more previous doses of Pfizer-BioNTech
COVID-19 Vaccine, NOT including at least 1
dose of 2024–25 COVID-19 vaccine
‡
Give 1 dose at least 8 weeks after the last dose.
3 or more previous doses of Pfizer-
BioNTech COVID-19 Vaccine, INCLUDING at
least 1 dose of 2024–25 COVID-19 vaccine
• These children may receive 1 additional dose
at least 8 weeks following the last
recommended dose.
‡
• Further additional dose(s) may be
administered, informed by the clinical
judgement of a healthcare provider and
personal preference and circumstances.
‡
• Any further additional doses should be
administered at least 8 weeks after the last
COVID-19 vaccine dose.
‡
* Children 6 months through 4 years of age should receive the same vaccine products for all doses.
† If mRNA vaccine is administered from different manufacturers, complete the recommended 3-dose series.
•
For people who received Dose 1 from one manufacturer but will receive subsequent dose(s) from a different manufacturer, administer:
•
Dose 2 at least 4 weeks after Dose 1.
•
Dose 3 at least:
8 weeks after Dose 2 for children 6 months through 4 years of age.
4 weeks after Dose 2 for people 5 years of age and older.
‡ In the following situations, a different age-appropriate COVID-19 vaccine product may be administered: the same vaccine is not available at the time of the clinic visit, the previous
dose is unknown, the person would otherwise not receive a recommended dose, or the person starts but is unable to complete a vaccination series with the same vaccine due to a
contraindication.
09/19/2024
CS321629-AV
8
2024–2025 COVID-19 Vaccine
Immunization Schedule
for People 6 Months of Age and Older
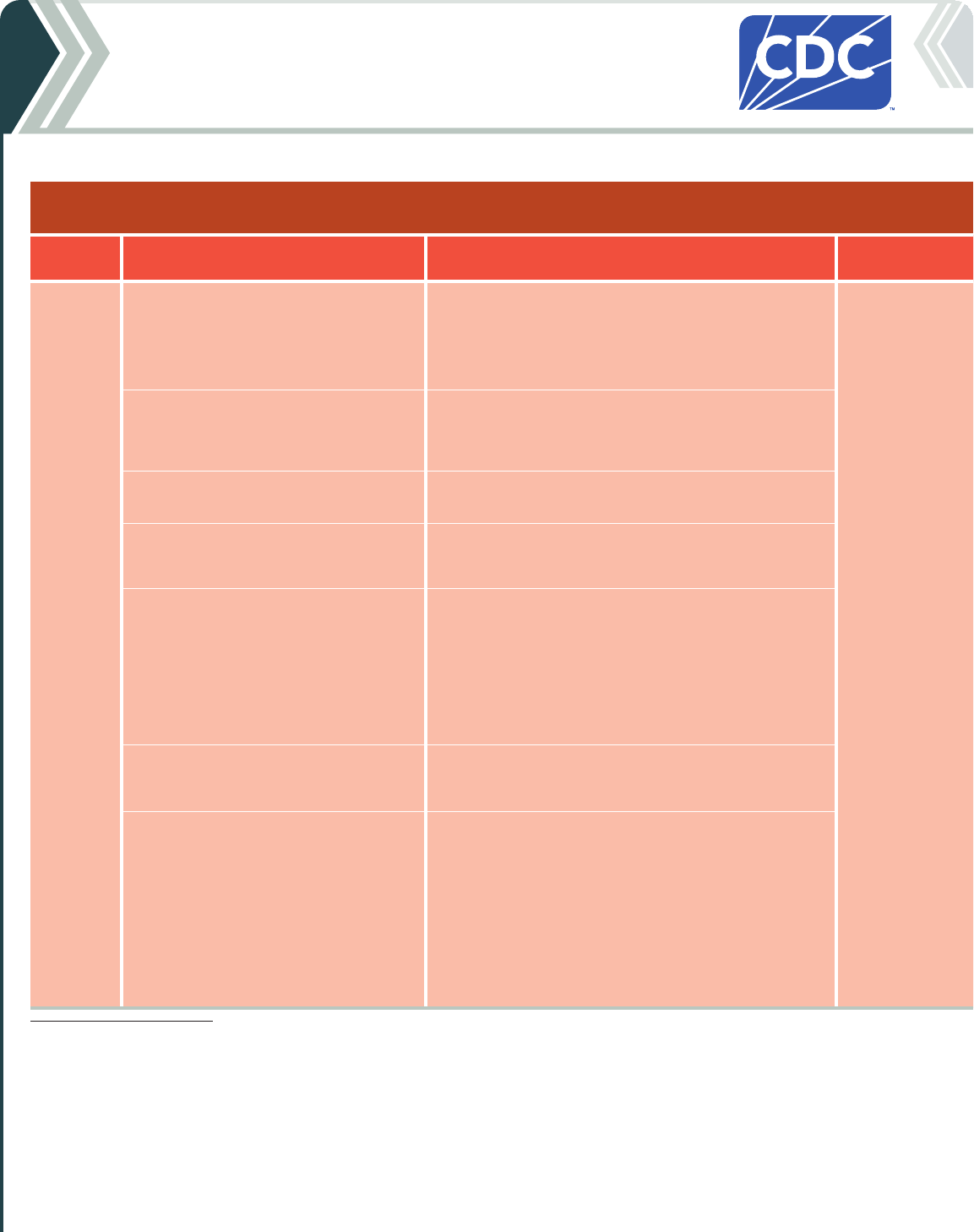
Table 2b. For people who ARE moderately or severely immunocompromised Continued
2024-25 Pfizer-BioNTech COVID-19 Vaccine
Vaccine type: mRNA | Do NOT use any previously available Pfizer-BioNTech COVID-19 vaccine products.
If current
age is:
And the COVID-19 vaccination history is: Then: Administer:
5 through
11 years
*†
Unvaccinated (0 doses)
Give a 3-dose initial series.
‡
• Dose 1 now.
• Dose 2 at least 3 weeks after Dose 1.
• Dose 3 at least 4 weeks after Dose 2.
0.3 mL/10 µg from
a blue-capped
single-dose vial
Intramuscular (IM)
injection
1 previous dose of any Pfizer-BioNTech
COVID-19 Vaccine (Dose 1)
§
Complete the series.
‡
Give:
• Dose 2 at least 3 weeks after Dose 1.
• Dose 3 at least 4 weeks after Dose 2.
2 previous doses of any Pfizer-BioNTech
COVID-19 Vaccine (Doses 1 and 2)
§
Complete the series.
‡
Give:
• Dose 3 at least 4 weeks after Dose 2.
3 or more previous doses of Pfizer-BioNTech
COVID-19 Vaccine, NOT including at least 1
dose of 2024–25 COVID-19 vaccine
Give 1 dose at least 8 weeks after the last dose
3 or more previous doses of Pfizer-
BioNTech COVID-19 Vaccine, INCLUDING at
least 1 dose of 2024–25 COVID-19 vaccine
• These children may receive 1 additional dose at
least 8 weeks following the last recommended
dose.
• Further additional dose(s) may be administered,
informed by the clinical judgement of a
healthcare provider and personal preference and
circumstances.
• Any further additional doses should be
administered at least 8 weeks after the last
COVID-19 vaccine dose.
* People 5 years of age and older should receive the same vaccine product to complete a 3-dose series. Additional doses may be any age-appropriate product.
† CDC recommends that people receive the age-appropriate vaccine product and dosage based on their age on the day of vaccination. Children who turn:
•
5 years of age during the 3-dose series should complete the series with age-appropriate dose 0.3 mL/10 µg .
•
12 years of age during the 3-dose series should complete the series with age-appropriate dose 0.5 mL/50 µg.
‡ In the following situations, a different age-appropriate COVID-19 vaccine product may be administered: the same vaccine is not available at the time of the clinic visit, the previous
dose is unknown, the person would otherwise not receive a recommended dose, or the person starts but is unable to complete a vaccination series with the same vaccine due to a
contraindication.
§ If mRNA vaccine is administered from different manufacturers, complete the recommended 3-dose series.
•
For people who received Dose 1 from one manufacturer but will receive subsequent dose(s) from a different manufacturer, administer:
•
Dose 2 at least 4 weeks after Dose 1.
•
Dose 3 at least:
8 weeks after Dose 2 for children 6 months through 4 years of age.
4 weeks after Dose 2 for people 5 years of age and older.
09/19/2024
CS321629-AV
9
2024–2025 COVID-19 Vaccine
Immunization Schedule
for People 6 Months of Age and Older
Table 2b. For people who ARE moderately or severely immunocompromised Continued
2024-25 Pfizer-BioNTech COVID-19 Vaccine - CONTINUED
Vaccine type: mRNA | Do NOT use any previously available Pfizer-BioNTech COVID-19 vaccine products.
If current
age is:
And the COVID-19 vaccination history
is:
Then: Administer:
12 years
and
older
*†
Unvaccinated 0 doses
Give a 3-dose initial series.
‡
• Dose 1 now.
• Dose 2 at least 3 weeks after Dose 1.
• Dose 3 at least 4 weeks after Dose 2.
0.3 mL/30 µg
from a
manufacturer-
filled syringe
Intramuscular
(IM) injection
1 previous dose of any Pfizer-BioNTech
COVID-19 Vaccine (Dose 1)
§
Complete the series.
‡
Give:
• Dose 2 at least 3 weeks after Dose 1.
• Dose 3 at least 4 weeks after Dose 2.
2 previous doses of any Pfizer-BioNTech
COVID-19 Vaccine (Doses 1 and 2)
§
Complete the series.
‡
Give:
• Dose 3 at least 4 weeks after Dose 2.
3 or more previous doses of any mRNA
COVID-19 Vaccine, NOT including at least
1 dose of 2024–25 COVID-19 vaccine
Give 1 dose at least 8 weeks after the last dose.
3 or more previous doses of any mRNA
COVID-19 Vaccine, INCLUDING at least 1
dose of 2024–25 COVID-19 vaccine
1 additional dose may be administered at least 8 weeks
following the last dose.
Further additional dose(s) may be administered,
informed by the clinical judgement of a health care
provider and personal preference and circumstances.
Administer additional doses at least 8 weeks after the
last dose.
2 or more doses of Novavax, NOT
including at least 1 dose of 2024–25
COVID-19 vaccine
Give 1 dose at least 8 weeks after the previous dose.
2 or more doses of Novavax,
INCLUDING at least 1 dose of 2024–25
COVID-19 vaccine
• People who are moderately or severely
immunocompromised may receive 1 additional dose
at least 8 weeks following the last recommended
dose.
• Further additional dose(s) may be administered,
informed by the clinical judgement of a health care
provider and personal preference and circumstances.
• Any further additional doses should be administered
at least 8 weeks after the last COVID-19 vaccine dose
* People 5 years of age and older should receive the same vaccine product to complete a 3-dose series. Additional doses may be any age-appropriate product.
† CDC recommends that people receive the age-appropriate vaccine product and dosage based on their age on the day of vaccination. Children who turn:
•
5 years of age during the 3-dose series should complete the series with age-appropriate dose 0.3 mL/10 µg .
•
12 years of age during the 3-dose series should complete the series with age-appropriate dose 0.5 mL/50 µg.
‡ In the following situations, a different age-appropriate COVID-19 vaccine product may be administered: the same vaccine is not available at the time of the clinic visit, the previous
dose is unknown, the person would otherwise not receive a recommended dose, or the person starts but is unable to complete a vaccination series with the same vaccine due to a
contraindication.
§ If mRNA vaccine is administered from different manufacturers, complete the recommended 3-dose series.
•
For people who received Dose 1 from one manufacturer but will receive subsequent dose(s) from a different manufacturer, administer:
•
Dose 2 at least 4 weeks after Dose 1.
•
Dose 3 at least:
8 weeks after Dose 2 for children 6 months through 4 years of age.
4 weeks after Dose 2 for people 5 years of age and older.

09/19/2024
CS321629-AV
10
2024–2025 COVID-19 Vaccine
Immunization Schedule
for People 6 Months of Age and Older
Table 2c. For people who ARE moderately or severely immunocompromised
2024-25 Novavax COVID-19 Vaccine
Vaccine type: Protein subunit | Do NOT use any previously available Novavax COVID-19 vaccine products.
If current
age is:
And the COVID-19 vaccination history is: Then: Administer:
12 years
and older
*
Unvaccinated (0 doses)
Give a 2-dose initial series.
• Dose 1 now.
• Dose 2 at least 3 weeks after Dose 1.
0.5 mL/5 µg rS protein
and 50 µg Matrix-M
adjuvant
in a manufacturer-filled
syringe (MFS)
Intramuscular (IM)
injectionw
1 previous dose of 2024-25 Novavax
COVID-19 Vaccine (Dose 1)
†
Give Dose 2 at least 3 weeks after the last dose.
1 or more previous doses of any COVID-19
vaccine, NOT including at least 1 dose of
2024–25 vaccine
Give 1 dose at least 8 weeks after the last dose.
2 or more previous doses of any COVID-19
vaccine, INCLUDING at least 1 dose of
2024–25 vaccine
1 additional dose may be administered at least
8 weeks following the last dose.
Further additional dose(s) may be
administered, informed by the clinical
judgement of a health care provider and
personal preference and circumstances.
Administer additional doses at least 8 weeks
after the last dose.
* Previously unvaccinated people should receive a 2-dose Novavax COVID-19 series. Additional doses may be any age-appropriate product.
† In the following situations, a different age-appropriate COVID-19 vaccine product may be administered: the same vaccine is not available at the time of the clinic visit, the previous
dose is unknown, the person would otherwise not receive a recommended dose, or the person starts but is unable to complete a vaccination series with the same vaccine due to a
contraindication.
