
V2.0
23-May-2023
Page 1 of 14
eReg Guidance Document
Review Sessions
Contents
Acronyms ........................................................................................................................................... 2
Review Sessions .............................................................................................................................. 2
eReg Access for External Reviewers ................................................................................................ 2
Creating a Review Session ............................................................................................................... 3
Configuring a Review Session .......................................................................................................... 4
Choosing Sections ........................................................................................................................ 4
Choosing Requirements ............................................................................................................... 5
Choosing Participating Sites ......................................................................................................... 6
Credentials and Regulatory Tracking Items ..................................................................................... 7
Deleting a Review Session ............................................................................................................... 7
SOP Review Sessions ..................................................................................................................... 8
Adding SOPs to Protocol Review Sessions ....................................................................................... 8
SOP Review Sessions ...................................................................................................................... 10
Creating an SOP Review Session .................................................................................................... 10
Configuring an SOP Review Session ............................................................................................... 12
Deleting an SOP Review Session .................................................................................................... 13
Viewing and Downloading Review Session Documents ..................................................... 13
Additional Resources.................................................................................................................... 14

V2.0
23-May-2023
Page 2 of 14
Acronyms
GCP: Good Clinical Practice
HRPP: Human Research Protection Program
IDS: Investigational Drug Service
IMV: Interim Monitoring Visit
SOP: Standard Operating Procedure
YCCI: Yale Center for Clinical Investigation
YNHHS: Yale New Haven Health System
Review Sessions
Review Sessions are used to provide External Reviewers, such as Monitors, Auditors and Inspectors,
electronic access to regulatory and essential documents and/ or Standard Operating Procedures (SOPs)
to conduct a review. Review Sessions can also be used to provide internal reviewers, such as site staff,
access to regulatory and essential documents and/ or SOPs.
eReg Access for External Reviewers
External Reviewers such as Monitors, Auditors and Inspectors must have an active eReg User Account to
be assigned and conduct a review session. External Reviewers will complete the User Access Request Form
located on the Yale Center for Clinical Investigation (YCCI) eReg website. External Reviewers are required
to complete eReg training in Advarra University prior to account activation. Ensure YCCI eReg Support
staff have sufficient time (approximately one week) to coordinate training and access for External
Reviewers.
The User Access Request Form requires a date of request, full name, email address, job title, relationship
to Yale/ Yale New Haven Hospital, Sponsor/ Home Organization and Role Requested. External Reviewers
will indicate they are External to Yale/ Yale New Haven Hospital on the request form. They will then select
a role of Monitor, Auditor or Inspector, as appropriate.
V2.0
23-May-2023
Page 3 of 14
The form must be completed by the External Reviewer as it includes an attestation. The attestation
indicates they have read and understand Yale University's Information Technology Appropriate Use Policy.
Site Staff who create review sessions should share this policy with their External Reviewers prior to
requesting they complete the User Access Request Form. External Reviewers must attest that they will
complete the role-appropriate eReg training prior to being granted access to the system. External
Reviewers must agree to use eReg only for work-related business, to not share their eReg password with
anyone and to not let anyone use eReg while logged into their account. External Reviewers must also
agree to only share the information obtained within eReg with personnel who have a legitimate work-
related need for the information.
Once an account is created, the External Reviewer will retain access to the system until eReg Support Staff
receives a request to inactivate the External Reviewer’s account or after 200 days of not logging into eReg,
whichever comes first. Should more than 200 days elapse between Review Sessions for an External
Reviewer or if the External Reviewer forgets their password, the account can be reactivated by contacting
eReg Support (eReg.Supp[email protected]u). Site Staff or the External Reviewer may make this request
directly.
Should an External Reviewer’s eReg access need to be terminated immediately for any reason, please
contact eReg Support (eReg.Support@yale.edu) for assistance. Site Staff who create the Review Session
can revoke access to regulatory and essential documents and/ or SOPs immediately by deleting the
Review Session. Refer to the Deleting a Review Session section below for more information on how to
delete a Review Session.
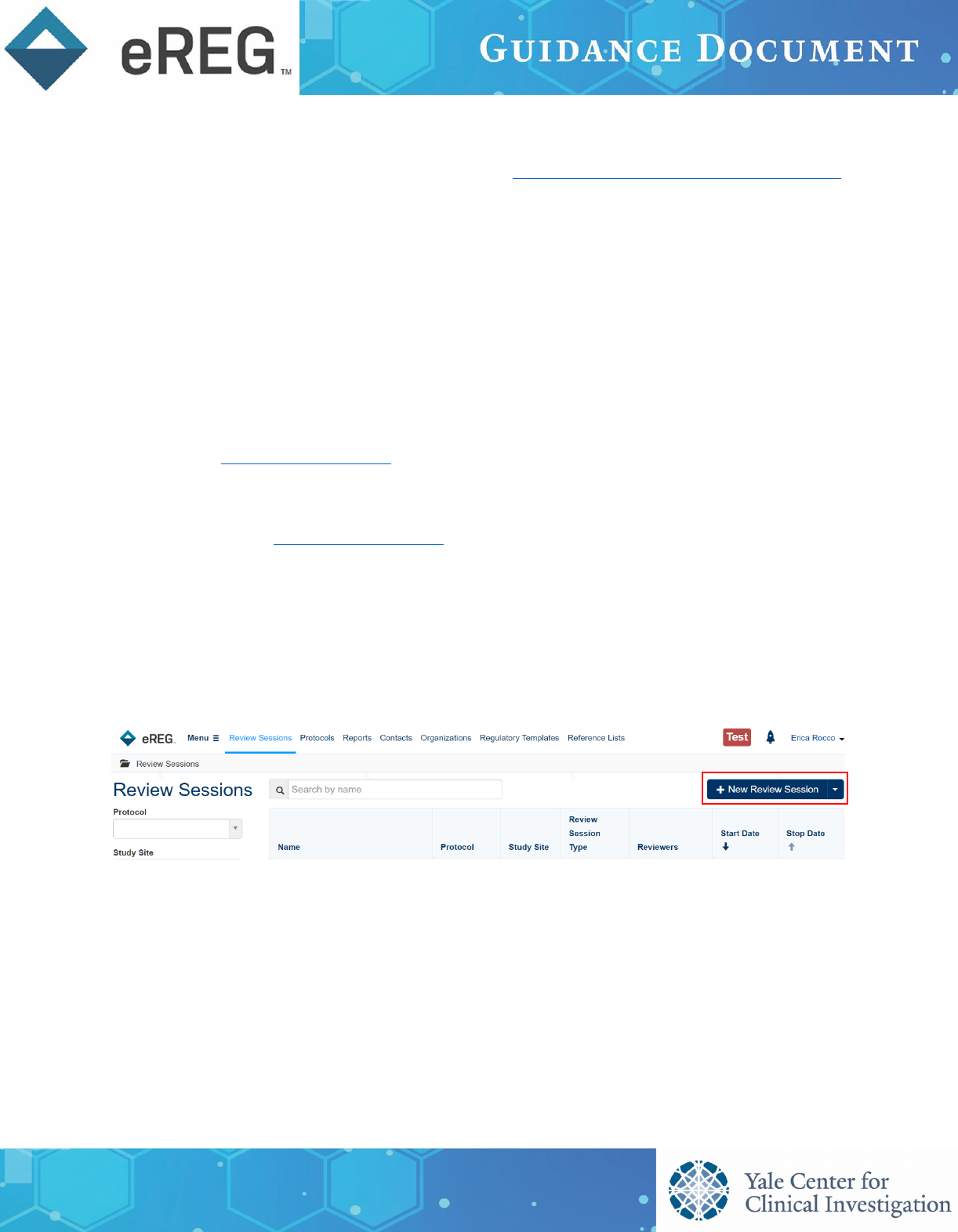
Creating a Review Session
On the Review Sessions landing page, click + New Review Session to open the Create Review Session pop-
up window.
In the Create Review Session pop-up window, complete the required fields which are marked with an
asterisk. Please note the Name of the session must be unique. We suggest including the review type and
a protocol identifier for the Name such as ‘Interim Monitoring Visit #[X] for [Protocol Identifier]’, ‘Site
Initiation Visit for [Protocol Identifier] or ‘Sponsor Audit for [Protocol Identifier]’.
A Stop Date for the Review Session is not required; however, we recommend entering a Stop Date so that
the Reviewer(s) does not have continuous access to the regulatory and essential documents for that
protocol and any SOPs included in the Review Session. If both a Start Date and Stop Date are entered for
the Review Session, the dates must be unique. A Review Session cannot be set up to start and stop on the
same day.

V2.0
23-May-2023
Page 4 of 14
Date ranges in eReg are based on midnight. If a reviewer is granted access September 30 – October 1, the
access will terminate at midnight on October 1. To ensure access through October 1, select an end date
of October 2.
Multiple reviewers can be assigned to one Review Session. After all fields are completed, click Create. The
action menu under the Create button will allow you to Create the existing entry and start a new entry.
Configuring a Review Session
After the Review Session is created, you will be brought to the Review Session configuration page. By
default, all sections and requirements (including any related documents) within the protocol are included
in the review session. If the reviewer(s) should only review select documents, you may customize the
sections and requirements available for review. If the reviewer(s) needs to review the complete eReg
binder, you do not need to customize the review session. In most cases, you would grant access to the
entire eReg Binder for Review Sessions. If the review session is being created for a multi-site protocol, all
participating sites are selected by default.
Choosing Sections
To customize Sections available for review, click the Choose Sections button.

V2.0
23-May-2023
Page 5 of 14
The Choose Sections pop-up window will open. Clear the checkboxes in front of the Protocol Sections you
do not want the reviewer to see in this Review Session. Then click Save.
Choosing Requirements
Within each Section, you may choose the Requirements you want the reviewer(s) to see in the Review
Session. When a Requirement is included in a Review Session, its associated documents (as well as any
related documents) are available to the reviewer. All Requirements within a Section are selected by
default to allow the reviewer to see all the regulatory and essential documents associated with that
Section.

V2.0
23-May-2023
Page 6 of 14
To customize Requirements available for review, click the Choose Requirements button in each Section.
The Choose Requirements pop-up window will open. To limit the requirements (and therefore the
documents) the reviewer(s) has access to, clear the checkboxes next to any Requirements that you do not
want to appear in the review session. Then, click Save.
Continue to configure the information and documents available to the reviewer in each section as
needed.
Choosing Participating Sites
If a Review Session is created for a multi-site protocol, you may choose the Participating Site files to
include in the Review Session.
To choose the Participating Sites to include, click Choose Participating Sites.

V2.0
23-May-2023
Page 7 of 14
To limit the Participating Sites the Reviewer has access to, clear the checkboxes next to any Participating
Sites that you do not want to appear in the Review Session. Then, click Save.
When finished choosing Sections, Requirements and/ or Participating Sites, click Back to Review Session
from the Actions button menu to preview the Review Session configured for the Reviewer.
Credentials and Regulatory Tracking Items
The credentials and regulatory tracking items that are included in the Review Session are based on the
protocol requirements and the documents’ valid date ranges. If a credential or regulatory tracking item’s
valid range does not overlap with when the staff member or organization was active on the protocol, that
document will not be included in the Review Session. Likewise, if a credential is not listed as a requirement
in the protocol outline, it will not appear in the Review Session regardless of the valid date range.
Deleting a Review Session
Review Sessions can be deleted. On the Review Sessions landing page, select the Review Session that
you wish to delete. Under the actions menu, select Delete.

V2.0
23-May-2023
Page 8 of 14
When Delete is clicked, you will be asked to confirm the delete. Click Delete to proceed with deleting the
Review Session. Click Cancel to retain the Review Session.
SOP Review Sessions
SOPs can be added to a Review Session created for a protocol. You can also create SOP-only Review
Sessions which allow a group of SOPs to be reviewed outside the context of a protocol.
Adding SOPs to Protocol Review Sessions
When configuring the Sections for your protocol Review Session, click the checkbox in front of SOPs to
add SOPs to the Review Session. Then click Save.

V2.0
23-May-2023
Page 9 of 14
Once the SOP section has been added, you will specify which SOPs you want to include by clicking on the
hyperlink within the SOP section.
When you open the hyperlink, click Choose SOPs to include specific SOPs in the Review Session.
Select from the list of SOPs available to you. This list will include all SOPs filed at the global level and any
department-specific SOPs to which you have access. Click Save.

V2.0
23-May-2023
Page 10 of 14
Once added, the Reviewer(s) will be able to view the SOPs. Please note that Reviewers’ access to SOPs is
limited to view-only. Reviewers do not have the ability to download SOPs. If the Reviewer requires a
copy of an SOP, you will need to provide it outside of eReg.
SOP Review Sessions
SOP Review Sessions are Review Sessions that only contain SOPs and are reviewed outside the context
of a protocol review. eReg users with a Regulatory Manager and/ or Multi-Site Department user role can
assign SOP Review Sessions to any eReg user (internal or external). For example, when a new staff
member joins the department, a Regulatory Manager in the department can assign them an SOP Review
Session in eReg and include all department-specific SOPs they need to review, aiding in the onboarding
process. Or, when a Sponsor Auditor is performing a Good Clinical Practice audit and needs to review
SOPs as part of the audit, the Regulatory Manager can assign them an SOP Review Session in eReg to
facilitate review of organizational and/ or departmental SOPs.
Creating an SOP Review Session
On the Review Sessions landing page, click the down arrow next to + New Review Session to select + New
SOP Review Session and open the Create SOP Review Session pop-up window.

V2.0
23-May-2023
Page 11 of 14
In the Create SOP Review Session pop-up window, complete the required fields which are marked with
an asterisk. Please note the Name of the session must be unique. We suggest including the review type
and relevant unique identifiers in the Name such as ‘SOP Review for GCP Audit for [Protocol Identifier]’ or
‘SOP Review for [First and Last Name] Onboarding’. Select a Review Session Type of SOP Only.
A Stop Date for the SOP Review Session is not required; however, we recommend entering a Stop Date so
that the Reviewer(s) does not have continuous access to the SOPs included in the Review Session. If both
a Start Date and Stop Date are entered for the SOP Review Session, the dates must be unique. An SOP
Review Session cannot be set up to start and stop on the same day.
Date ranges in eReg are based on midnight. If a reviewer is granted access September 30 – October 1, the
access will terminate at midnight on October 1. To ensure access through October 1, select an end date
of October 2.

V2.0
23-May-2023
Page 12 of 14
Multiple reviewers can be assigned to one SOP Review Session. After all fields are completed, click Create.
The action menu under the Create button will allow you to Create the existing entry and start a new entry.
Configuring an SOP Review Session
After the SOP Review Session is created, you will be routed to the SOP Review Session configuration page.
Click Choose SOPs to select the SOPs to be included in the SOP Review Session.
Select from the list of SOPs available to you. This list will include all SOPs filed at the global level and any
department-specific SOPs to which you have access. Click Save.
Once added, the Reviewer(s) will be able to view the SOPs. Please note that Reviewers’ access to SOPs is
limited to view only. Reviewers do not have the ability to download SOPs. If the Reviewer requires a
copy of an SOP, you will need to provide it outside of eReg.
When finished choosing SOPs, click Back to Review Session from the Actions button menu to preview the
SOP Review Session configured for the Reviewer.

V2.0
23-May-2023
Page 13 of 14
Deleting an SOP Review Session
SOP Review Sessions can be deleted. On the Review Sessions landing page, select the SOP Review
Session that you wish to delete. Under the actions menu, select Delete.
When Delete is clicked, you will be asked to confirm the delete. Click Delete to proceed with deleting the
SOP Review Session. Click Cancel to retain the SOP Review Session.
Viewing and Downloading Review Session Documents
Within a Review Session, the Reviewer can view and download protocol documents, view signature
information for documents that were electronically signed within eReg, and view SOPs if included in the
Review Session.
To download documents into a file folder for review outside of eReg, click Download Documents from the
Actions button menu. Sections will display as main folders and Requirements as sub-folders within the
relevant Section. If the delegation of authority log is maintained within eReg, a current version of the log
will populate and be filed within the Delegation of Authority folder. Folders and sub-folders will not be
created for any Sections and Requirements that are blank, i.e., no documents filed within that Section or
Requirement or for Sections that are view-only within eReg, i.e., SOPs.

V2.0
23-May-2023
Page 14 of 14
If new protocol documents are uploaded to the Sections and Requirements included in a Review Session,
the Review Session will reflect the new documents in real time. However, the downloaded documents
are representative of what was available for review in the eReg binder at the time of download.
Additional Resources
• Electronic Regulatory Management System Access Guide
• eReg Learning Portal
• YCCI eReg website: https://medicine.yale.edu/ycci/researchservices/systems/ereg/
