
65;(5(%,*/0)9(9@65;(5(%,*/0)9(9@
0.0;(364465:65;(5(%,*/0.0;(364465:65;(5(%,*/
,636.0*(35.05,,905. (*<3;@$*/63(9:/07
9,(;05.(2,:-964!7,5"0;05,:"96*,::,:(5+9,(;05.(2,:-964!7,5"0;05,:"96*,::,:(5+
65:0+,9(;065:47/(:0:65 69;/,955=09654,5;:65:0+,9(;065:47/(:0:65 69;/,955=09654,5;:
/90:;67/,9(4465:
65;(5(%,*/
,: (990:
(4,:(:;96
",;,96;;
9<*,&(55(
6336>;/0:(5+(++0;065(3>692:(;/;;7:+0.0;(3*64465:4;,*/,+<.,63',5.9
"(9;6-;/,5=09654,5;(3$*0,5*,:64465:,636.@64465:(5+;/,0505.5.05,,905.
64465:
#,*644,5+,+0;(;065#,*644,5+,+0;(;065
(4465:(990: (:;966;;"(5+(55(&9,(;05.3(2,:-96467,570;
405,:796*,::,:(5+*65:0+,9(;065:>0;/,47/(:0:65569;/,95,5=09654,5;:(5%,*/#,70:/
8<(;$*00?7
%/0:9;0*3,0:)96<./;;6@6<-69-9,,(5+67,5(**,::)@;/,(*<3;@$*/63(9:/07(;0.0;(364465:65;(5(
%,*/;/(:),,5(**,7;,+-6905*3<:06505,636.0*(35.05,,905.)@(5(<;/690A,+(+4050:;9(;696-0.0;(3
64465:65;(5(%,*/69469,05-694(;06573,(:,*65;(*;:1<:20,>0*A4;,*/,+<

9,(;05.(2,:-964!7,5"0;05,:"96*,::,:(5+65:0+,9(;065:47/(:0:9,(;05.(2,:-964!7,5"0;05,:"96*,::,:(5+65:0+,9(;065:47/(:0:
65 69;/,955=09654,5;:65 69;/,955=09654,5;:
):;9(*;):;9(*;
9,(;05.(2,:-964!7,5"0;05,:"96*,::,:(5+65:0+,9(;065:47/(:0:65 69;/,95
5=09654,5;:%/0:+6*<4,5;:<44(90A,:;/,30;,9(;<9,6-40505.70;3(2,:;/96<./>0;/(
7(9;0*<3(9-6*<:650::<,:;/(;(9,302,3@;6),6-:7,*0(39,3,=(5*,;6;/,*9,(;065(5+4(5(.,4,5;6-70;
3(2,:05569;/,95*304(;,:"0;3(2,:(9,:0473@>(;,9)6+0,:-694,+)@C3305.;/,67,570;3,-;<765;/,
*6473,;0656-40505.67,9(;065:>0;/>(;,902,5(;<9(33(2,:40505.70;3(2,:+0:73(@(/<.,+0=,9:0;@05
,(*/6-;/,:,:<)1,*;(9,(:6>,=,970;3(2,:(9,@6<5.(5+;/,9,-69,(9,;@70*(33@05(565,8<030)90<4
:;(;,>0;/9,:7,*;;6;/,099(;,6-C3305.>(;,98<(30;@(5+)0636.@$,7(9(;,:,*;065:+,(3>0;/+0--,9,5;
(:7,*;:6-70;3(2,:05*3<+05.;/,094697/64,;9@.,636.@/@+96.,636.@.,6*/,40:;9@(5+)0636.@
,7,5+05.65;/,;@7,(5+36*(;0656-;/,405,;/,9,4(@),67769;<50;0,:;6,5/(5*,;/,9,*9,(;065(369
,*636.0*(3),5,C;:6-(.0=,570;3(2,-69,?(473,)@9,3(5+:*(705.(5+9,=,.,;(;05.;/,:/69,305,)@
(++05.,5.05,,9,+/()0;(;-69(8<(;0*30-,(5+4(05;(0505.>(;,98<(30;@%/,*9,(;0656-(70;3(2,4(@),
(9,.<3(;69@9,8<09,4,5;;640;0.(;,,5=09654,5;(3047(*;:-96440505.67,9(;065:(5+69),05*3<+,+
(:7(9;6-(*36:<9,(5+9,*3(4(;06573(5
(:,+657<)30:/,+*(:,:;<+0,:6-70;3(2,:3(9.,:*(3,)06,5.05,,905.7961,*;:/(=,/(+40?,+
:<**,::*64465*65:,5:<:0:;/(;4(507<3(;0656-70;3(2,*/,40:;9@0:+0D*<3;,?7,5:0=,(5+;(2,:
4(5@@,(9:;6(*/0,=,9,4,+0(;065.6(3:69;/0:9,(:650;0:79<+,5;;6;(2,:;,7:;/96<./6<;405,
67,9(;065;69,+<*,;/,302,30/66+6--<;<9,>(;,98<(30;@796)3,4:<765*36:<9,3:60;4(2,::,5:,;6
,5.05,,9;/,3(2,05:<*/(>(@;/(;0;>033(*/0,=,0;:4(?04(3,5+<:,76;,5;0(3>/,;/,90;),7,94(5,5;
(5+:(-,:;69(.,6-405,>(:;,/()0;(;-69(8<(;0*30-,9,*9,(;06569>(;,9:<773@
,@>69+:,@>69+:
"0;3(2,:9,*3(4(;06540505.C://()0;(;>(;,98<(30;@
0:*07305,:0:*07305,:
5=09654,5;(3$*0,5*,:B,636.@B0505.5.05,,905.
644,5;:644,5;:
(5(+0(5%,*/50*(3#,769;6-0:/,90,:(5+8<(;0*$*0,5*,:%/0:9,769;0:(*67@6-(56D*0(3
>6927<)30:/,+)@;/,6=,954,5;6-(5(+((5+>(:56;796+<*,+05(D30(;065>0;/69>0;/;/,
,5+69:,4,5;6-;/,6=,954,5;6-(5(+(
9,(;0=,64465:0*,5:,9,(;0=,64465:0*,5:,
%/0:>6920:30*,5:,+<5+,9(9,(;0=,64465:;;90)<;065 65*644,9*0(3 6,90=(;0=,&692:
0*,5:,
%/0:(9;0*3,0:(=(03()3,(;0.0;(364465:65;(5(%,*//;;7:+0.0;(3*64465:4;,*/,+<.,63',5.9
Creating lakes from open pit mines: processes and
considerations, with emphasis on northern
environments
Christopher H. Gammons
1
, Les N. Harris
2
, James M. Castro
3
, Peter A.
Cott
4
, and Bruce W. Hanna
4
1
Montana Tech of The University of Montana
Butte, Montana 59701
2
University of British Columbia
Vancouver, British Columbia V6T 1Z4
3
Montana Department of Environmental Quality
Helena, Montana 59620
and
4
Department of Fisheries and Oceans
Yellowknife, Northwest Territories X1A 1E2
2009
Canadian Technical Report of
Fisheries and Aquatic Sciences 2826
Canadian Technical Report for Fisheries and Aquatic Sciences 2826
2009
Creating lakes from open pit mines: processes and considerations, with emphasis on
northern environments
By
Christopher H. Gammons
1
, Les N. Harris
2
, James M. Castro
3
, Peter A. Cott
4
, and Bruce
W. Hanna
4
1
Montana Tech of The University of Montana
Butte, Montana 59701
2
University of British Columbia
Vancouver, British Columbia V6T 1Z4
3
Montana Department of Environmental Quality
Helena, Montana 59620
and
4
Department of Fisheries and Oceans
Yellowknife, Northwest Territories X1A 1E2
ii
© Her Majesty the Queen in Right of Canada, 2009.
Cat. No. Fs 97-6/2826E ISSN 0706-6457
Correct citation for this publication:
Gammons, C. H., Harris, L.N., Castro J.M., Cott, P.A., and Hanna, B.W. 2009. Creating
lakes from open pit mines: processes and considerations - with emphasis on northern
environments. Can. Tech. Rep. Fish. Aquat. Sci. 2826: ix + 106 p.
iii
TABLE OF CONTENTS
List of tables........................................................................................................................ v
List of figures...................................................................................................................... v
Abstract............................................................................................................................viii
1. Introduction..................................................................................................................... 1
2. Characteristics of pit lakes............................................................................................. 3
2.1 Types of mining pit lakes.......................................................................................... 3
2.2 Hydrogeology of pit lakes........................................................................................ 5
2.3 Limnology of pit lakes............................................................................................. 8
2.3.1 Vertical structure and classification.................................................................. 8
2.3.2 Predicting lake turnover.................................................................................. 10
2.3.3 Landslides and lake turnover .......................................................................... 12
2.3.4 Positive and negative consequences of lake turnover...................................... 12
2.3.5 Brine exclusion and thermohaline convection................................................ 13
2.3.6 Double-diffusion convection .......................................................................... 15
2.4 Chemistry of pit lakes............................................................................................ 17
2.4.1 General trends in pit lake chemistry ................................................................ 17
2.4.2 Review of chemical processes ......................................................................... 18
2.4.2.1 Evaporation................................................................................................... 20
2.4.2.2 Photosynthesis............................................................................................... 21
2.4.2.3 Other photochemical reactions ..................................................................... 22
2.4.2.4 Leaching of soluble salts............................................................................... 22
2.4.2.5 Precipitation of hydrous ferric oxide ............................................................ 23
2.4.2.7 Subaqueous pyrite oxidation......................................................................... 26
2.4.2.8 Bacterial reduction of iron and sulfate.......................................................... 27
2.5 Biology of pit lakes................................................................................................ 29
2.5.1. Bacteria .......................................................................................................... 30
2.5.2. Plankton .......................................................................................................... 31
2.5.3 Macrophytes..................................................................................................... 32
2.5.4. Invertebrates................................................................................................... 33
2.5.5. Fish................................................................................................................. 33
2.5.5.1. Metal toxicity and fish ................................................................................ 34
2.5.6. Waterfowl ....................................................................................................... 35
2.6. Unique aspects of pit lakes in northern environments.......................................... 36
3. pit Lake modeling and prediction ................................................................................ 40
3.1 Experimental procedures ....................................................................................... 40
3.1.1 Acid-base accounting (ABA) tests.................................................................. 40
3.1.2 Kinetic tests..................................................................................................... 41
3.1.3 Pit lake mesocosm experiments...................................................................... 42
3.2 Computer modeling ............................................................................................... 42
3.2.1. Hydrogeological models ................................................................................. 42
3.2.2 Limnological models ...................................................................................... 43
3.2.3 Geochemical models........................................................................................ 43
3.2.4 Biological models ........................................................................................... 44
iv
4. Enhancing beneficial end uses for pit lakes.................................................................. 44
4.1 Summary of beneficial end uses ............................................................................ 45
4.1.1 Storage of mine waste..................................................................................... 45
4.1.2 Water supply................................................................................................... 47
4.1.3 Ecological Habitat........................................................................................... 47
4.1.4 Aquaculture..................................................................................................... 49
4.1.5 Recreation and tourism ................................................................................... 49
4.1.6 Metal recovery ................................................................................................ 51
4.1.7 Heat pump applications................................................................................... 51
4.1.8 Scientific study................................................................................................ 52
4.2 Strategies for enhancing end uses.......................................................................... 52
4.2.1 Landscaping and backfilling........................................................................... 52
4.2.2 Rapid flooding ................................................................................................ 52
4.2.3 Chemical Treatment........................................................................................ 53
4.2.4 Biological Treatment ..................................................................................... 56
4.2.4.1 Aerobic biological treatment........................................................................ 56
4.2.4.2 Anaerobic biological treatment.................................................................... 57
4.2.5 Establishment of fisheries.................................................................................. 59
4.2.5.1 Fish introduction.......................................................................................... 59
4.2.5.2. Fisheries habitat creation and enhancement ................................................ 60
4.2.5.3. Potential fish species.................................................................................... 64
5. Case studies and examples........................................................................................... 65
5.1 Kimberlite Diamond Mines .................................................................................... 65
5.1.1 Kimberlite geology ......................................................................................... 65
5.1.2. Kimberlite hydrogeology............................................................................... 66
5.1.3. Chemistry of groundwater associated with kimberlite deposits ..................... 67
5.1.4. Implications to diamond mine pit lakes in northern Canada .......................... 68
5.2 Colomac gold mine, Northwest Territories ........................................................... 69
5.3. East Pit coal mine, Alberta..................................................................................... 71
5.4 Iron Ore Mines, Minnesota and Ontario................................................................ 73
5.5 Sleeper Gold Mine, Nevada.................................................................................... 74
5.6. Anchor Hill, South Dakota .................................................................................... 76
6. Acknowledgments........................................................................................................ 78
7. References cited........................................................................................................... 79
8. Glossary of terms and acronyms................................................................................... 96
v
LIST OF TABLES
Table 1. Summary of chemical processes occurring in pit lakes. The information is
arranged from top to bottom in the approximate order of increasing depth in the
lake............................................................................................................................ 19
Table 2. (Continued from previous page)......................................................................... 20
Table 3: A list of some common sulfide minerals and their likelihood to generate acid on
weathering................................................................................................................. 41
Table 4. Summary of beneficial end uses for pit lakes, with examples........................... 45
LIST OF FIGURES
Figure 1. Aerial view of open pit diamond mines in the Northwest Territories, Canada.. 1
Figure 2. Comparison of the shape and size of selected pit lakes (drawn to scale from
Google Earth images). The maximum depths of some of the larger lakes are also
given. All of the lakes shown on the right from Plessa, Germany are filling former
lignite strip mines, and are relatively shallow (most < 10 m)..................................... 4
Figure 3. Example calculations showing the rate of filling of a pit lake by groundwater.
Q
gw
is the flux of groundwater entering the lake. ....................................................... 6
Figure 4. Schematic diagrams comparing: a) a terminal lake, and b) a flow-through lake.
Dashed lines on the right are groundwater table contours. Arrows are groundwater
flow lines..................................................................................................................... 7
Figure 5. Density of water at different temperatures and salinities. The dashed line
shows the maximum density at any given salinity. The freezing point depression
(solid arrow) is approximate, and depends on the composition of the dominant salts
in the water.................................................................................................................. 8
Figure 6. Schematic diagrams comparing: a) a holomictic lake, and b) a meromictic lake.
Arrows denote zones of seasonal mixing.................................................................... 9
Figure 7. Salt deposits forming in shallow ice on the surface of the Berkeley pit lake
(March, 2002). The concentric salt bands are roughly 10 to 50 meters in diameter.
The deposits were not analyzed for mineralogy, but are believed to be gypsum and
other metal sulfate salts. (Photo: James Madison, Montana Bureau of Mines and
Geology). .................................................................................................................. 13
Figure 8. Schematic diagram showing thermohaline convection in a high-latitude
meromictic lake (after Gibson, 1999a). Cold brines form during salt exclusion at the
ice-water interface and sink through the water column. Warmer, less dense water
rises to take its place. The maximum depth of penetration of the sinking brine flow
may change from year to year................................................................................... 14
Figure 9. Hypothetical seasonal changes in water density profiles for a high-latitude
meromictic lake (modified from Gibson, 1999b). THC refers to thermohaline
convection................................................................................................................. 15
Figure 10. Illustrations of the double-diffusion convection process. a) Because the rate
of diffusion of heat is much faster than salt, the upper water layer warms up and
rises while the lower water sinks; b) Schematic diagram showing characteristic
“stairstep” profiles in water temperature and salinity that can set up in a lake
undergoing double-diffusion convection. ................................................................. 16
vi
Figure 11. Summary of chemical processes occurring in mining pit lakes. .................... 18
Figure 12. Eh-pH diagram showing the general conditions where Fe is soluble (unshaded
areas) and insoluble (shaded areas). The diagram was drawn for 10 mg/L Fe, 10
mg/L C, and 100 mg/L S........................................................................................... 24
Figure 13. Adsorption of selected cationic trace metals onto hydrous ferric oxide as a
function of pH. Diagram modified from Drever (1997).......................................... 25
Figure 14. Bowen’s Reaction Series, showing the sequence of crystallization of common
silicate minerals from magma. Minerals near the top of the series are furthest from
equilibrium with the Earth’s surface and will chemically weather at a faster rate... 26
Figure 15. Diagram showing the approximate Eh and pH conditions of selected bacterial
reactions that may be important in mining pit lakes. All data used to draw this
diagram were taken from Drever (1997). Fe and Mn reduction lines assume
equilibrium with ferrihydrite and birnessite, respectively. Upper and lower limits
for Fe and Mn reduction were taken at 1 µmol/L and 1 mmol/L dissolved Fe
2+
and
Mn
2+
, respectively. Upper and lower limits for sulfate reduction were taken at SO
4
2-
/(H
2
S+HS
-
) ratios of 1000 and 1, respectively. Upper and lower limits for nitrate
reduction were taken at NO
3
-
= 0.1 mmol/L, and P(N
2
) = 0.001 and 1 bar,
respectively. .............................................................................................................. 28
Figure 16. Ranges in published data for LC
50
concentrations of selected heavy metals to
fishes (from Kelley, 1999)........................................................................................ 35
Figure 17. Schematic diagram of a pit lake in a permafrost setting. Conduction of heat
from the deep lake may gradually melt back the permafrost layer, resulting in
decreased slope stability and slope failure. Lakes that penetrate the permafrost layer
may also serve as conduits for deep groundwater recharge or discharge, depending
on local hydrogeological conditions. (The sketch depicts groundwater discharge).39
Figure 18. Diagram showing different possibilities for backfilling a mining pit on a
hillside: a) complete backfill; b) partial backfill with a shallow lake; c) no backfill
with a deep lake. The shaded region in “c” shows a wedge of permanently
dewatered bedrock where acid mine drainage is likely to occur. ............................. 46
Figure 19. Big horn sheep (Ovis Canadensis) inhabiting a reclaimed open pit gold mine
in Montana (photo by Chris Gammons, 2007). ........................................................ 48
Figure 20. The “Big Hole” pit lake at Kimberley, South Africa. (Source: Wikipedia.org;
photo by Rudolph Botha, March, 2005). .................................................................. 50
Figure 21. Photographs of lime treatment sludge: a) plume of hydrous ferric oxide in the
surface of the Berkeley pit lake formed by disposal of alkaline lime treatment sludge
(photo by Chris Gammons, June, 2006); b) “green rust” formed by rapid pH increase
of Fe
II
-rich mine water (Chris Gammons). ............................................................... 55
Figure 22. The dependence of the solubility of selected metal-hydroxide minerals on pH.
The “optimal treatment” line is generalized. Water quality standards vary for each
metal and from region to region. Note the high solubility of Fe
2+
relative to Fe
3+
. In
mine waters, Fe
2+
concentrations may be controlled by another less soluble mineral,
such as “green rust” or siderite (FeCO
3
). Diagram modified from Nordstrom and
Alpers (1999). ........................................................................................................... 56
Figure 23. Examples of fish habitat creation and enhancement techniques as described in
Section 4.2.5.2. Artificial reef created with rubble and waste-rock (A), floating reef-
raft (B), large boulders for cover (C), the planting of littoral (D) and riparian
vii
vegetation (E), submerged conifers for providing cover (F), tires and donated
concrete (G) and the addition of large woody debris (H). ........................................ 60
Figure 24. Simplified geology of a kimberlite pipe......................................................... 65
Figure 25. East Pit Lake, Alberta was formed through the reclamation of a surface pit left
over from a dragline coal mining operation.............................................................. 72
Figure 26. Photograph of the swimming beach at the Portsmouth Mine Pit Lake,
Minnesota (taken from Wikipedia.org)..................................................................... 74
Figure 27. Addition of methanol to the Anchor Hill pit lake. (Photo by Brian Park, May,
2001). ........................................................................................................................ 77
viii
ABSTRACT
Gammons, C. H., Harris, L.N., Castro J.M., Cott, P.A., and Hanna, B.W. 2009. Creating
lakes from open pit mines: processes and considerations - with emphasis on northern
environments. Can. Tech. Rep. Fish. Aquat. Sci. 2826: ix + 106 p.
This document summarizes the literature of mining pit lakes (through 2007), with a
particular focus on issues that are likely to be of special relevance to the creation and
management of pit lakes in northern climates. Pit lakes are simply waterbodies formed
by filling the open pit left upon the completion of mining operations with water. Like
natural lakes, mining pit lakes display a huge diversity in each of these subject areas.
However, pit lakes are young and therefore are typically in a non-equilibrium state with
respect to their rate of filling, water quality, and biology. Separate sections deal with
different aspects of pit lakes, including their morphometry, geology, hydrogeology,
geochemistry, and biology.
Depending on the type and location of the mine, there may be opportunities to enhance
the recreational or ecological benefits of a given pit lake, for example, by re-landscaping
and re-vegetating the shoreline, by adding engineered habitat for aquatic life, and
maintaining water quality. The creation of a pit lake may be a regulatory requirement to
mitigate environmental impacts from mining operations, and/or be included as part of a
closure and reclamation plan.
Based on published case studies of pit lakes, large-scale bio-engineering projects have
had mixed success. A common consensus is that manipulation of pit lake chemistry is
difficult, expensive, and takes many years to achieve remediation goals. For this reason,
it is prudent to take steps throughout mine operation to reduce the likelihood of future
water quality problems upon closure. Also, it makes sense to engineer the lake in such a
way that it will achieve its maximal end-use potential, whether it be permanent and safe
storage of mine waste, habitat for aquatic life, recreation, or water supply.
Key words: Pit lakes, reclamation, mining, fish habitat, water quality
RÉSUMÉ
Gammons, C. H., Harris, L.N., Castro J.M., Cott, P.A. et Hanna, B.W. 2008. La création
de lacs dans les mines à ciel ouvert : processus et points à examiner – en mettant l’accent
sur les environnements du Nord. Rapp. tech. can. sci. halieut. aquat. 2826: ix + 106 p.
Le présent document résume la documentation des lacs miniers (jusqu’en 2007), en
mettant un accent particulier sur les enjeux susceptibles d’être particulièrement pertinents
à la création et à la gestion des lacs miniers dans les climats du Nord. Les lacs miniers
ix
sont tout simplement des plans d’eau formés en remplissant d’eau le puits à ciel ouvert
laissé à la fin des opérations minières. À l’instar des lacs naturels, les lacs miniers
présentent une immense diversité dans chacun de ces domaines. Cependant, les lacs
miniers sont jeunes et donc généralement dans un état hors d’équilibre en ce qui a trait à
leur taux de remplissage, la qualité de leur eau et leur biologie. Des sections distinctes
traitent de différents aspects des lacs miniers, notamment leur morphométrie, leur
géologie, leur hydrogéologie, leur géochimie et leur biologie.
Selon le type et l’emplacement de la mine, il pourrait y avoir des possibilités d’améliorer
les avantages récréatifs ou écologiques d’un lac minier, par exemple en refaisant
l’aménagement paysager et en remettant en végétation le rivage, en ajoutant un habitat
aménagé pour la vie aquatique et en maintenant la qualité de l’eau. La création d’un lac
minier pourrait être une exigence réglementaire afin d’atténuer les répercussions des
opérations minières sur l’environnement ou faire partie intégrante d’un plan de fermeture
et de remise en état.
Selon des études de cas publiées au sujet des lacs miniers, les projets de bio-ingénierie de
grande envergure ont connu certains succès. On s’entend pour dire que la manipulation
de la chimie d’un lac minier est difficile et coûteuse et qu’il faut de nombreuses années
pour atteindre les objectifs de restauration. Pour cette raison, il est prudent de prendre des
mesures pendant l’opération minière pour réduire la probabilité de futurs problèmes de
qualité de l’eau à la fermeture de la mine. De plus, il est logique d’aménager le lac de
façon qu’il atteigne son potentiel maximal d’utilisation finale, que ce soit le stockage
permanent et sécuritaire des déchets miniers, un habitat pour la vie aquatique, récréative
ou l’approvisionnement d’eau.
Mots clés : lacs miniers, remise en état, exploitation minière, habitat du poisson, qualité
de l’eau

1
1. INTRODUCTION
Exploration and development of non-renewable natural resources is occurring rapidly in
northern Canada (Cott et al., 2003; Birtwell et al., 2005; Sibley et al., 2008) with some of
the most prolific industry sectors being diamond and metal mining (Birtwell et al., 2005).
A common extraction method for these resources is open pit mining (Fig. 1). Open-pit
mining results in residual pits, overburden or waste rock piles, and sometimes tailings
impoundments left on the landscape. The excavated pits are of various depths and sizes,
but all require environmental reclamation. One possible reclamation endpoint could be
the creation of end-pit lakes.
Figure 1
. Aerial view of open pit diamond mines in the Northwest Territories, Canada.
Pit lakes are formed by water filling the open pit left upon the completion of mining
operations. These pits can be filled by artificially flooding or allowing the pits to fill
naturally through hydrological processes such as precipitation or ground water infiltration
(Castro and Moore, 2000). For example, a gravel quarry pit that has filled with rain water
would be one form of pit lake. Pit lakes may have long-term benefits as a water source
for industrial activities rather than relying on natural systems (Cott et al., 2008a).
Depending on water quality, it may also be possible to modify or enhance pits to create
aquatic habitat for fish and wildlife.
Often environmental regulations require that closure and reclamation plans are developed
by proponents in the initial stages of environmental assessment and updated on a regular
basis as the project proceeds through the regulatory phase (INAC, 2007). Other
regulatory instruments, such as Canada’s Fisheries Act administered by the Department
2
of Fisheries and Oceans (DFO), require that fish habitat lost, for example due to mining
operations, be compensated by the creation of new fish habitat or enhancement of
existing habitat (DFO, 1986). However, the lack of information on pit lake creation, from
a biological perspective, makes it difficult for government regulators and resource
managers to make informed decisions. In the Northwest Territories (NWT), knowledge
gaps regarding pit lakes have been recognized as a major issue by regulatory agencies,
proponents and other interested stakeholders such as Aboriginal groups during the review
of large mining operations.
As Birtwell et al. (2005) identified, there are also many information gaps regarding
habitat requirements for fish species in the north that would make development of viable
fish habitat in a pit lake all the more challenging. Very few projects - of any type -
intended to create or enhance fish habitat have been properly documented (Smokorowski
et al., 1998, Quigley et al., 2006). In the north the paucity of information is even more
pronounced with only two fish habitat enhancement projects in the NWT having been
assessed and published (see Jones et al., 2003; Cott, 2004). Documentation on the
successful creation of pit lakes as self sustaining aquatic ecosystems is scarce,
particularly for northern environments. Information on the creation of pit lakes as a
result of diamond mining activities in the NWT is absent because none of the active
diamond mines have been closed. Proactive planning is required to make the best use of
these pits if they are to be made into pit lakes.
This report is an effort to address some of these data gaps regarding the state of
knowledge on pit lakes as part of environmental reclamation activities. Although the
impetus for this report was related to the current need for information to properly address
pit lake issues in northern Canada, it was recognized that many characteristics of pit lakes
are similar regardless of their geographical location or what resource is being extracted.
Therefore, the scope of the paper provides a representative overview of pit lakes in
general, with an emphasis on northern environments.
Section 2 of this report describes the characteristics of pit lakes in terms of their
morphometry, geology, hydrogeology, limnology, chemistry, and biology. This section
concludes with a description of some unique aspects of pit lakes in northern
environments and considerations for their development. Section 3 briefly summarizes
some numerical models currently available that can be used to predict the internal
dynamics of pit lakes. Section 4 deals with methods to enhance pit lakes depending on
what are deemed to be their most beneficial end uses, from storage of mine wastes to
being platforms for limnological study. Section 5 includes several case studies of mining
pit lakes in North America, including a review of the literature on mine waters associated
with kimberlites and diamond mines. For reader convenience, a glossary including
acronyms and technical terms is included following the references cited.
Rather than an exhaustive review of all literature pertaining to pit lakes, this report is
meant to be an overview of relevant information on the creation of pit lakes as
ecologically functioning systems. The information herein can be used as a resource for
3
the varied stakeholders that could be involved in the development or review of projects
that include the creation of pit lakes.
2. CHARACTERISTICS OF PIT LAKES
2.1 TYPES OF MINING PIT LAKES
By definition a pit lake is a lake that forms by flooding of an excavated mining pit. Other
mine impoundments, such as tailings ponds, are not included in this definition. Different
kinds of mines produce pits with various physical, geochemical, and ecological
characteristics. Pits from mining of chemically inert materials tend to mirror the
geochemistry of their surroundings, and lakes that form in such pits do not commonly
present environmental problems. Their value as resources for recreation, fisheries, water
supply, and wildlife habitat depends mostly on their topography, their safety
characteristics (susceptibility to slides, rock falls, etc.), their hydrological characteristics
(interaction with groundwater and surface waters and the quality of both), and climate.
These inert materials include sand, gravel, clay (e.g., bentonite), limestone, talc, iron ore,
and bauxite, among others.
Pits from other types of mining are more strongly affected by the chemistry of the mined
resources. Silver and many nonferrous base metals such as copper occur as sulfide ores
(Guilbert and Park, 1986). Many gold deposits are also rich in sulfides. Sulfide ore
deposits commonly contain pyrite (FeS
2
), which reacts with atmospheric oxygen and
water to generate sulfuric acid according to the following equation (Nordstrom and
Alpers, 1999):
FeS
2
+ 15/4O
2
+ 7/2H
2
O → Fe(OH)
3
(s) + 2SO
4
2–
+ 4H
+
(1)
Coal deposits, except for those with very low sulfur contents, commonly contain
significant levels of pyrite or its polymorph, marcasite. Organic-rich shales that are
mined for phosphate fertilizer may also contain pyrite. Thus, mines of metals, industrial
minerals, and solid fuels can all be potential sources of acid waters. Uranium deposits
may or may not contain significant pyrite, but the radionuclides from the ore can present
their own problems in pit lake water.
Gemstone deposits can occur in a wide variety of rock types or in unconsolidated
sediments. Most of these deposits are mined as placers or in small mines that do not
result in large pits at the end of mining. Diamond deposits are an important exception to
this rule. Because of the high value of diamonds and the fact that they occur in fairly
extensive bodies of kimberlite, diamond mines commonly produce deep pits and
therefore potential pit lakes.
Besides the geochemical differences among types of pit lakes, the lakes from different
mine types can also vary physically. Surface mines producing metal ores tend to be
shaped like their ore bodies. Some are more or less circular in plan view, while others

4
may have rectangular or irregular shapes (Fig. 2). What they all have in common is a
tendency to be smaller at their bottoms than at their tops because of the need to build haul
roads on the walls to reach the bottom. This produces a pit lake with little or no shallow
littoral zone unless steps are taken to produce shallow zones in the pit after mining.
Figure 2. Comparison of the shape and size of selected pit lakes (drawn to scale from
Google Earth images). The maximum depths of some of the larger lakes are also given.
All of the lakes shown on the right from Plessa, Germany are filling former lignite strip
mines, and are relatively shallow (most < 10 m).
Because kimberlite pipes are deep and generally have more or less cylindrical shapes,
diamond pits tend to assume the shapes of steep-sided cones. In this respect they are
similar to many metal-mine pits. Examples of such pits are the ones at the Ekati and
Diavik mines in the Northwest Territories (Nowicki et al., 2004; Donnelly et al., 2007).
In contrast, open-pit coal mines (strip mines) on relatively level terrain commonly use
area-mining techniques, in which overburden is stripped off and coal is mined along a
linear front. As the mine front advances, overburden is used to reclaim mined-out parts
of the pit. At the end of the mine life, this commonly leaves a long, narrow linear pit
lake, which sometimes is referred to as a “final cut lake.” It is common for such a lake to
have a steep highwall or cliff along its front side and a more gradual slope on the back
side. The depth of the lake is the sum of the thicknesses of the overburden and the coal
seam. However, other shapes are possible for coal pits (see Fig. 2).
The water in coal pit lakes can range from extremely acidic to somewhat alkaline,
depending on the chemistry of the local groundwater, the coal bed, and the country rock.
In a study of coal pit lakes in Montana, the Dakotas, and Wyoming, Anderson and
Hawkes (1985) found the quality of water did not differ significantly from that of water
in bentonite pits or stock-watering ponds
.
On the other hand, water in lakes formed from
abandoned high-sulfur coal or lignite mines can be strongly acidic. Extensive strip
mining of lignite deposits in Lusatia, Germany, has left a legacy of over 500 pit lakes
Sleeper, NV
Yerington,
NV
Dexter,
NV
Anchor
Hill, SD
Elizabeth, VT
1 km
240 m
Berkeley, MT
Plessa, Germany
ML 111
ML 110
ML 112
ML 107
ML 116
ML 117
ML 118
Island Copper, BC
382 m
110 m
14 m
Portsmouth, MN
137 m
105 m
5
(Geller et al., 1998; Friese et al., 1998). Many of these lakes are strongly acidic (pH < 3).
These lakes tend to be very large in surface area but relatively shallow, being for the most
part < 10m in average depth. The cluster of pit lakes near Plessa is a good example (see
Fig. 2). German scientists and engineers have used the acid pit lakes (in particular,
Mining Lake 111, Fig. 2) as a natural laboratory to test different in situ and ex situ
technologies for treatment and remediation. Many of these treatment concepts have
focused on bioremediation, for example, through artificial stimulation of bacterial sulfate
reduction.
2.2 HYDROGEOLOGY OF PIT LAKES
Hydrogeology determines how rapidly open pit mines fill with water after closure, and
also influences the final steady state water budget of the lake that is formed. Most large
open pits will eventually intersect the water table during mining, and consequently steps
must be taken to dewater the pit during operation. After mining ceases, the dewatering
pumps are usually shut down, allowing the water tables to rebound and – in some cases –
a pit lake to form. A generalized water balance equation for a pit lake can be summarized
as follows:
P + SW
in
+ GW
in
= E + (T) + SW
out
+ GW
out
± ΔS (2).
The definition of the terms in equation (2) are as follows: P is direct precipitation falling
on the surface of lake; SW
in
is the sum of any surface water inputs such as diverted
streams, stormflow, pit-wall runoff, or waste water being disposed of in the lake; GW
in
is
groundwater entering the lake; E is evaporation; T is plant transpiration (in parentheses
because it is expected to be a minimal term for most pit lakes); SW
out
is surface water
exiting the system (including water that is pumped, treated, and discharged to receiving
water bodies); GW
out
is groundwater exiting the lake; and ΔS is the change in storage,
i.e., the volume of water in the lake. Terms on the left side of equation (2) are water
inputs, whereas terms on the right side (with exception of ΔS) are water outputs. If input
> output, then ΔS is positive, and the lake volume will increase.
Left to their own devices, large open pit lakes can take a very long time (decades to
centuries) to fill with water. This is particularly true in arid or semi-arid areas where P
and SW
in
are minimal. The rate of groundwater input varies quite a bit from mine to
mine, and depends on the site geology, topography, and climate. A rough estimate of
GW
in
can be obtained by noting the amount of water that was pumped during active
mining operations. Thus, if the dewatering pumps were removing 15,000 m
3
/day on
average during the mine life, then the rate of flooding from groundwater rebound will be
around 15,000 m
3
/day, at least during the early stages of flooding. However, this rate
will change as the pit fills with water, as can be deduced from Darcy’s Law:
Q
gw
= K·A·(Δh/Δl) (3)
where Q
gw
is the flux of groundwater into the lake (e.g., m
3
/day), K is the hydraulic
conductivity (e.g., m/day) of the fractured bedrock or overburden, A is the cross-sectional

6
area of the flooded portion of the lake, and Δh/Δl is the hydraulic gradient, or steepness
of the water table in the zone of groundwater capture surrounding the lake. As the pit
floods and the surface of the lake rises, the hydraulic gradient will decrease. However,
this effect is offset by the fact that the value of A increases with time as the volume of the
lake increases. This is because groundwater enters the lake from all sides below the
water line, not just from the bottom or top. The net result of these offsetting factors is
that the filling rate of a pit lake may actually increase with time during the initial period
of flooding, but will eventually level off and then slowly decrease to zero as the lake
surface approaches its final equilibrium elevation. Figure 3 illustrates this concept. For
these calculations, a hypothetical pit lake was assumed with the following characteristics:
1) a pit geometry equal to a right-circular cone with a radius twice its height; 2) fractured
bedrock with K = 0.1 m/day; and 3) a change in elevation between the steady state water
table surface and the bottom of the pit of 200 m. Following these assumptions, the
maximum groundwater flux into the lake occurs ~ 25 years after flooding begins. Figure
3 also shows that the depth of the lake increases rapidly at first, but then levels off as the
total volume of the lake increases and as Q
gw
diminishes to zero.
0
50
100
150
200
250
0 25 50 75 100 125 150
time (years)
Depth of lake, m
0
400
800
1200
1600
2000
Groundwater flux (m
3
/day)
lake depth
Q
gw
Figure 3. Example calculations showing the rate of filling of a pit lake by groundwater.
Q
gw
is the flux of groundwater entering the lake.
Once a pit lake has filled to its ultimate surface elevation, ΔS is zero (barring seasonal
changes), and the input and output terms in equation (2) will cancel. There are now many
different hydrogeological scenarios for the lake, and these are important factors to
consider for “end use” decisions. For example, in an arid climate such as Nevada, it is
very possible that evaporation will completely balance any water input terms, such that
SW
out
and GW
out
are both zero at steady state. Such a lake is referred to as a “terminal”
lake (Fig. 4a). A “flow-through” lake, on the other hand, will receive groundwater inputs
from one side, but will lose groundwater to the other side (Fig. 4b). Flow-through lakes

7
are especially common when the pit was excavated on a hillside with an initially sloping
water table. Finally, if water inputs are high (such as in a wet climate, or where a stream
is permanently diverted into the lake), then the final lake will probably have a surface
water output. This could either be an engineered spillway or stream, or lake water that is
pumped and (possibly) treated before discharging to a receiving stream or lake.
Figure 4. Schematic diagrams comparing: a) a terminal lake, and b) a flow-through lake.
Dashed lines on the right are groundwater table contours. Arrows are groundwater flow
lines.
In some cases an open pit mine will be connected to underground mine workings. This is
often the case for old mining districts where access to high-grade mineralization was first
developed by vertical mine shafts. Later on, with the development of new technologies
to beneficiate lower and lower grade ore bodies, much of the “in between” ore would
then be mined by open pit. A good example of this situation is the Butte mining district
in Montana. Beginning in the 1870s and continuing through the mid-1970s, miners dug
over 16,000 km of underground passageways, including over 50 shafts, many of which
were more than 1 km deep. Between 1956 and 1982, the Berkeley open pit mine
excavated a very large hole right in the middle of this labyrinth of tunnels and shafts.
The majority of the underground mine openings are now flooded, and the local hydraulic
gradients are such that most of this water is slowly heading towards the Berkeley pit lake.
In terms of hydrogeology, this complicates the picture considerably over what would be
the case for a mine void that is simply filling with groundwater from fractured bedrock.
Whereas some of the mine tunnels in Butte were backfilled with waste, many were not,
and the latter most likely act as “plug-flow” corridors for rapid groundwater conveyance.
During the early period of mine flooding, secondary salts that had accumulated in the
mine tunnels over decades of active mining were flushed into the pit lake, contributing to
a. Terminal lake
b. Flow-through lake

8
the poor quality of the lake (Maest et al., 2004). Interestingly, the temperature of the
majority of the mine shaft water in Butte is elevated (typically in the range 15 to 35°C,
see Gammons et al., 2006a) compared to the adjacent groundwater (~ 10°C). The influx
of this warm water into the deep lake is one reason for the fact that temperatures in the
deep water (currently between 8 and 9°C) are higher than would be expected for a natural
lake in Montana.
2.3 LIMNOLOGY OF PIT LAKES
2.3.1 Vertical structure and classification
Of fundamental importance to any pit lake study is information on the vertical structure
of the lake and whether it is conducive to seasonal overturn. Most natural lakes in
northern climates are holomictic, meaning that they typically undergo a complete top-to-
bottom turnover during the Spring and Fall when surface water temperatures pass through
their density maximum of ~ 4°C (Fig. 5). Holomictic lakes become vertically stratified
in the summer, with a warmer, less dense “epilimnion” resting on top of a colder, denser
“hypolimnion” (Fig. 6a). These two layers are separated by a thermocline (also known as
the “metalimnion”), i.e., a zone of rapid temperature change with depth. In winter,
temperatures < 4°C in the shallowest water (often under ice cover) again result in
density-stratification. Rather than being a static horizontal plane, internal waves of large
amplitude and period can set up at the thermocline interface, being driven by surface
waves, episodic influx of groundwater, or landslides (Stevens and Lawrence, 1998;
Hodges et al. 2000; Wetzel, 2001).
Figure 5. Density of water at different temperatures and salinities. The dashed line
shows the maximum density at any given salinity. The freezing point depression (solid
arrow) is approximate, and depends on the composition of the dominant salts in the
water
.
994
996
998
1000
1002
1004
1006
1008
1010
-5 0 5 10 15 20 25 30 35
Tem
p
erature
,
o
C
Density, kg/m
3
TDS
(mg/L)
f
r
e
e
z
i
n
g
ρ
max
10000
7500
5000
2500
0

9
Figure 6. Schematic diagrams comparing: a) a holomictic lake, and b) a meromictic lake.
Arrows denote zones of seasonal mixing.
In contrast to holomictic lakes, meromictic lakes contain a bottom layer of highly saline
water than has a density too great to mix with overlying waters, regardless of the
temperature profile. Figure 6b shows a typical meromictic lake with a shallow
epilimnion, a mid-level hypolimnion and a deep monimolimnion (after Boehrer and
Schultze, 2006). Although the epilimnion and hypolimnion may mix seasonally (together
these zones are referred to as the “mixolimnion”), water in the monimolimnion is too
dense and salty to overturn. The interface between the mixolimnion and monimolimnion
is often referred to as a “chemocline” (i.e., an interface in chemical composition or
salinity). Chemoclines can also coincide with temperature gradients. It is not uncommon
for salty water in the monimolimnion of a meromictic lake to have a temperature well
above 4°C, whereas the temperature of the overlying hypolimnion is typically colder and
closer to the maximum density value (Stevens and Lawrence, 1998; Gammons and
Duaime, 2006). Heat can be added to the bottom water either from the surrounding rocks
(most continental settings have geothermal gradients of ~ 20 to 35°C/km) or from
influent warm ground water, or both. In general, the effect of changes in salinity is
greater than the effect of changes in temperature when determining the density of water
in a pit lake. As shown in Figure 5, if the salinity of the deep water exceeds that of the
epilimnion
hypolimnion
monimolimnion
thermocline
chemocline
b. Meromictic lake
epilimnion
hypolimnion
thermocline
a. Holomictic lake
mixolimnion

10
overlying water column by 2500 mg/L, and if the hypolimnion is at the maximum density
corresponding to 4°C, then turnover cannot occur until the temperature of the
monimolimnion is raised above 20°C. Barring the existence of anomalous geothermal
activity, such warm temperatures are extremely unlikely for deep lake waters.
Limnological textbooks (e.g., Wetzel, 2001) have different terms for meromictic lakes,
depending on the dominant mechanism that leads to density stratification. Ectogenic
meromixis occurs when some external or human-induced event brings saline water into a
freshwater lake, or fresh water into a saline lake. A good example is the Island Copper
pit lake of Vancouver Island, B.C., which was flooded to > 90% volume with ocean
water, and then capped with a fresh surface water layer (Fisher and Lawrence, 2006).
Crenogenic meromixis occurs when high-salinity groundwater flows into deep parts of a
lake. The Brenda pit lake near Kelowna, B.C. (Stevens and Lawrence, 1998) and the
McLaughlin pit lake in California (Rytuba et al. 2000), are two examples. Biogenic
meromixis occurs when a biological process increases the salinity of the monimolimnion.
This situation may occur in pit lakes with high sedimentation rates of organic carbon
combined with abundant hydrous oxides of Fe and/or Mn. In the anoxic bottom waters of
the lake and underlying sediment, bacteria reductively dissolve the hydrous metal oxides,
increasing the concentration of dissolved Fe and Mn. This process has been documented
from Fe-rich natural lakes in northern Canada (Campbell and Torgensen, 1980). Finally,
cryogenic meromixis occurs in some lakes or fjords at high latitude with thick, seasonal
ice cover. Salt exclusion during crystallization of ice creates brine streamers that descend
by gravity, sometimes to the bottom of the lake (see section 2.3.5). Thawing of ice in the
summer adds low-salinity water to the lake surface, further stabilizing the density
stratification.
2.3.2 Predicting lake turnover
Mining pit lakes with high depth to width ratio are less apt to undergo complete lake
turnover (Doyle and Runnells, 1997; Castro and Moore, 2000). The aspect ratio of a
given pit lake can be quantified by Z
r
, otherwise known as the “relative depth”
(Hutchinson 1957):
Z
r
(%) =
A
π
⋅⋅
m
Z50 (4)
where Z
m
is the maximum depth, and A is the surface area of the lake. In a survey of
mining pit lakes around the world, Doyle and Runnells (1997) suggested that most
artificial lakes with Z
r
> 25% are meromictic, although there were a few lakes with Z
r
>
25% that were holomictic, and many lakes with Z
r
< 25% that were meromictic. Clearly
there are other factors which dictate whether a given pit lake is permanently stratified.

11
A more useful parameter in predicting lake turnover is the Wedderburn Number (Stevens
and Lawrence, 1997, 1998):
L
g'h
W
2
*
2
e
u
=
(5)
where h
e
is the depth (m) of the surface layer (epilimnion), L is the fetch, or length of the
surface of the lake (m), g' is the modified gravity of the interface between the surface and
underlying water layers, and
∗
u
is the friction velocity. Values of W (a dimensionless
number) > 1 indicate a lake with stable stratification that is unlikely to mix, whereas
values < 1 indicate instability and a greater likelihood of turnover. The modified gravity
term is given by the following equation:
()
0
12
-
'
ρ
ρρ
⋅
=
g
g
(6)
where g = 9.8 m/s
2
,
1
ρ
and
2
ρ
are the densities of the epilimnion and the underlying
hypolimnion, respectively, and
0
ρ
is the reference density, usually taken as the average
of
1
ρ
and
2
ρ
. The friction velocity term is given as:
2
1
2
10
0
D
C u
⎟
⎟
⎠
⎞
⎜
⎜
⎝
⎛
=
∗
U
a
ρ
ρ
(7)
where C
D
is the drag coefficient (a dimensionless number with a value near 10
-3
for
water),
a
ρ
is the air density (1.2 kg/m
3
for dry air at 20°C and sea level), and U
10
is the
wind velocity (m/s) measured 10 m above the water surface. Combining the preceding 3
equations yields:
()
L C
- h
W
a
2
10D
12
2
e
ρ
ρρ
U
g
=
(8)
From equation (8), it follows that the probability of turnover increases as the epilimnion
becomes thinner, as the density contrast between the surface and underlying water layers
diminishes, as the wind speed increases, as the length of the lake increases, or as the
density of air increases. Note that the effects of h
e
and U
10
are second-power. The
a
ρ
term changes slightly with changes in temperature or relative humidity, and is effected to
a greater extent by changes in altitude. Thus, wind at high altitude where the air is
thinner has less force than wind of the same velocity at sea level.

12
2.3.3 Landslides and lake turnover
Because open pit mines are typically steep-sided and barren of vegetation with highwalls
composed of highly fractured and blasted bedrock, slope stability is always a major
concern. The rebound in groundwater pressure after mine closure may actually increase
the probability of a major slide by decreasing the effective stress borne by the rock
matrix. After a mining pit is flooded, a large landslide has several potentially serious
consequences: 1) possible threat to human health or damage to buildings or roads near the
affected pit highwall; 2) drowning of individuals or wildlife on the shore of the lake due
to “tidal waves”; and 3) possible inducement of turnover due to turbulent mixing. The
latter scenario is of particular relevance to the present discussion, because it brings up the
risk that a pit lake designed to be meromictic may nonetheless undergo a landslide-
induced mixing event. Such an event occurred in the Berkeley pit lake of Butte,
Montana, in September 1998, when a portion of the southeast highwall failed, sending an
estimated 130,000 m
3
of poorly consolidated sediment and waste rock into the lake
(Gammons and Duaime, 2006). A slope failure of this magnitude or larger could
completely mix a pit lake that otherwise would be density-stratified. As another
example, Pieters and Lawrence (2007) mention a smaller rock slide event that may have
resulted in mixing of deep water in the Colomac Zone 2 pit lake.
2.3.4 Positive and negative consequences of lake turnover
Lake turnover may be a desirable or undesirable occurrence, depending on the end use of
the pit lake in question. If a lake is dimictic (overturn in spring and fall) or polymictic
(frequent overturns throughout the year) then the major element chemistry will be more
or less evenly distributed with depth, and there is also a good chance that the entire water
column will be oxygenated. This is good from the standpoint of supporting benthic
organisms and fishes at different depths in the lake. However, because mine pits often
contain potentially hazardous solid materials, there is also a chance that contaminants
will be leached from weathered bedrock or mine waste on pit walls or in sediment and re-
distributed to shallow waters. As well, infrequent turnover events may allow time for
chemical or biological reactions to transform the water quality of the hypolimnion, which
could have negative consequences when the lake undergoes a major turnover event.
These include upwelling of water with low (or no) DO and upwelling of water rich in
nutrients (such as ammonia, phosphate, or DOC). A sudden drop in DO could result in a
fish kill, whereas a sudden influx in nutrients could result in eutrophication and blooms
of algae or cyanobacteria (Wetzel, 2001). Finally, if the upwelling water has high
concentrations of dissolved gas, such as carbon dioxide (CO
2
), hydrogen sulfide (H
2
S) or
methane (CH
4
), then these gases may exsolve or possibly even bubble out of the water,
creating an O
2
-depleted air column immediately above the lake surface. This
phenomenon, sometimes referred to as a “limnic eruption”, has been documented from a
few natural lakes (such as Lake Nyos, a volcanic crater lake in Cameroon), but is less
likely to occur in mining pit lakes (Murphy, 1997).

13
2.3.5 Brine exclusion and thermohaline convection
When the surface of a lake freezes, most of the dissolved salts are excluded from the ice
crystals that are formed. In rare cases, the rejected salt may accumulate on the surface of
the ice, forming interesting patterns (Fig. 7). More commonly, the salt (in the form of a
dense brine droplet with low freezing point) will burrow its way downwards by gravity
through the ice and thence to the underlying water. If the lake has moderate or high
salinity to begin with, then salt exclusion during rapid ice growth can create high-density
brine flows that descend by gravity through the water column (Fig. 8). For example,
Wakatsuchi (1984) conducted benchtop experiments that showed “brine streamers”
originating near the bottom of growing ice sheets. Wakatsuchi argued that this process
likely takes place in the polar oceans where cold brines may drop appreciable distances
(up to 400 m) before mixing with less dense seawater. Meanwhile, lower salinity water
must rise to occupy the volume left behind. The overall process is a localized form of
thermohaline convection, which is more familiar to most people as the process that drives
the global circulation of water in the world’s oceans.
Figure 7. Salt deposits forming in shallow ice on the surface of the Berkeley pit lake
(March, 2002). The concentric salt bands are roughly 10 to 50 meters in diameter. The
deposits were not analyzed for mineralogy, but are believed to be gypsum and other
metal sulfate salts. (Photo: James Madison, Montana Bureau of Mines and Geology).

14
Figure 8. Schematic diagram showing thermohaline convection in a high-latitude
meromictic lake (after Gibson, 1999a). Cold brines form during salt exclusion at the ice-
water interface and sink through the water column. Warmer, less dense water rises to take
its place. The maximum depth of penetration of the sinking brine flow may change from
year to year.
Gibson (1999b) described thermohaline convection from lakes in Antarctica, and these
processes may have relevance to mining pit lakes in northern Canada. Figure 9 shows in
more detail how vertical changes in salinity of an ice-covered water body can develop
with changing seasons. During the summer the lake is strongly stratified with an
unfrozen, low-salinity epilimnion, a colder and intermediate-salinity hypolimnion, and a
high salinity monimolimnion (Fig. 9a). With the onset of winter ice formation, excluded
salt creates brines that circulate through the top layer of water, increasing the salinity of
the epilimnion (Fig. 9b, 9c). This continues until later in the winter when the salinity of
the epilimnion approaches that of the hypolimnion, at which point these two layers will
mix (Fig. 9d) and any new brine streamers formed by salt exclusion can now descend all
the way to the monimolimnion. Later in the year when the ice cover melts, a cap of low
salinity water once again forms on the lake, and the process repeats itself.
ice
sinking
brine
monimolimnion
mixolimnion

15
Figure 9. Hypothetical seasonal changes in water density profiles for a high-latitude
meromictic lake (modified from Gibson, 1999b). THC refers to thermohaline
convection.
2.3.6 Double-diffusion convection
Double-diffusion convection is an interesting limnological phenomenon that can set up in
a meromictic lake when colder, lower-salinity water sits on top of warmer, higher-salinity
water. In this situation, there is a net upwards diffusive flux of both heat and dissolved
salt at the interface between the two water layers. However, the rate of diffusion of heat
is roughly 100 times faster than the rate of diffusion of salt. Thus, the water immediately
overlying the density interface (or pyncnocline) will warm up faster than it will become
saltier, and the water immediately below will cool down faster than it will become less
saline. The net result is that the upper parcel of water will rise (but only a short distance),
whereas the lower parcel of water will sink (Fig. 10a). What results is a series of
horizontal layers of vertically-mixed water separated by multiple pyncnoclines. Lakes or
ocean waters undergoing double-diffusion convection exhibit a characteristic “staircase”
vertical profile in salinity and temperature (Schmid et al., 2004) (Fig. 10b).
THC
THC
THC
wave
mixing
Salinity
monimolimnion
hypolimnion
epilimnion
a. Summer
b. Early winter
c. Mid-winter d. Late winter/early spring
ice
ice
ice
monimolimnion
hypolimnion
Salinity
Depth
Depth

16
Figure 10. Illustrations of the double-diffusion convection process. a) Because the rate
of diffusion of heat is much faster than salt, the upper water layer warms up and rises
while the lower water sinks; b) Schematic diagram showing characteristic “stairstep”
profiles in water temperature and salinity that can set up in a lake undergoing double-
diffusion convection.
Although all meromictic lakes must undergo diffusion of dissolved solids and heat across
the pyncnocline, this does not necessarily result in double-diffusion convection. To
maintain meromictic conditions, the density difference due to salinity gradient must be
greater than the density difference due to the temperature gradient. Turner (1965)
developed a “density ratio” term R
p
, which is given by:
T
S
Δ⋅
Δ⋅
=
α
β
R
p
(9)
where S is the salinity, T is the temperature,
β
is the coefficient of saline contraction
(0.78 kg/m
3
/psu at 10°C), and
α
is the coefficient of thermal expansion (- 0.15 kg/m
3
/°C
at 10°C). [Note: 1 psu ~ 1000 mg/L of dissolved solid]. When R
p
is >> 1, then the
boundary separating the two layers is stable. Conversely, when R
p
is < 1, the boundary is
unstable, and the lake will overturn. Double-diffusion convection is most likely to occur
when 1 < R
p
< 10, in other words, when conditions are favorable for meromixis, but just
barely.
Although double-diffusion convection has been documented from some natural
meromictic lakes (e.g., Lake Nyos, Cameroon, see Schmid et al., 2004), the authors are
not aware of a mining pit lake where this has been observed. Unless a very detailed CTD
(conductivity-temperature-depth) profile was obtained during field sampling, the
relatively subtle “staircase” changes in salinity and temperature (Fig. 10b) could easily go
unnoticed.
heat
flux
salt
flux
heat
flux
salt
flux
cooler,
less salty
water
warmer,
saltier
water
pyncnocline
cooler,
less salty
water
warmer,
saltier
water
pyncnocline
salinity
temperature
T, S
depth
a.
b.

17
2.4 CHEMISTRY OF PIT LAKES
2.4.1 General trends in pit lake chemistry
Mining pit lakes display a wide diversity in water quality, reflecting the wide diversity in
the style of mineral deposits that were extracted. General trends in pit lake chemistry
have been reviewed by several workers (Davis and Eary, 1997; Friese et al., 1998; Eary,
1999; Castro and Moore, 2000). Many pit lakes from former gold, metal-sulfide, or coal
mines contain elevated concentrations of heavy metals (e.g., Al, Cd, Cu, Fe, Mn, Ni, Pb,
Zn, U) and/or metalloids (e.g., As, Sb, Se, Te) that can pose a threat to the environment.
In contrast, pit lakes formed from mining of iron ore, gravel, or industrial minerals (e.g.,
talc, asbestos) may have quite low concentrations of dissolved metals. Depending on the
end-use of a particular pit lake, other water quality parameters may also become
important, such as salinity, turbidity, DO concentration, and nutrient content.
The single most important parameter that controls pit lake water quality is pH. This is
because the mobility of most metals and metalloids is strongly pH-dependent, for reasons
that are elaborated in the following sections. In addition, most aquatic life forms have
relatively narrow ranges of pH tolerance. As shown by Davis and Eary (1997), pit lakes
that are strongly acidic (pH < 4) tend to have high concentrations of “cationic” trace
metals, such as Al
3+
, Cu
2+
, Fe
2+
/Fe
3+
, Mn
2+
, and Zn
2+
, in addition to high concentrations
of the common cations Ca
2+
, Mg
2+
, Na
+
and K
+
. In low-pH lakes, the dominant anion is
usually sulfate (SO
4
2-
or HSO
4
-
). In contrast, pit lakes that have near-neutral to alkaline
pH tend to have relatively low concentrations of cationic trace metals, and may contain
appreciable bicarbonate (HCO
3
-
) in addition to dissolved sulfate. Alkaline lakes may be
highly elevated in metalloids, such as As or Se, present as dissolved anions such as
HAsO
4
2-
or SeO
4
2-
(Davis and Eary, 1997). Although these general trends exist, each
mineral deposit is different and needs to be evaluated on a case-by-case basis. For
example, pit lakes formed by flooding of uranium deposits may be rich in dissolved U
and Th.
Through a combination of leaching of soluble salts on mine walls and evapo-
concentration, most pit lakes – regardless of pH – have relatively high total dissolved
solids (TDS). Elevated turbidity (i.e., total suspended solids, or TSS) is also a common
characteristic of mining pit lakes, due to rapid physical erosion of steep mine walls
combined with wave action along shorelines during stormy periods. Another common
source of turbidity is oxidation of dissolved iron to hydrous ferric oxide (HFO), a red-
brown substance that is very fine-grained and slow to settle by gravity (see section
2.4.2.5). Turbidity can block sunlight, resulting in reduced rates of photosynthesis.
DO is essential for many forms of aquatic life, in particular fishes. Most pit lakes will
contain elevated DO levels in shallow water, due to free gas exchange with the
atmosphere coupled with wave-mixing. However, the deeper zones of many pit lakes are
susceptible to depletions in DO, and this is exacerbated by the often higher chemical
oxygen demand of mining vs. natural lakes. For example, oxidation of pyrite and other
metal sulfides on submerged mine walls provides a long-term sink for dissolved oxygen

18
in the hypolimnion or monimolimnion of stratified pit lakes. Another important sink for
DO is oxidation of dissolved ferrous iron (Fe
2+
).
In terms of nutrient concentrations, most pit lakes are naturally oligotrophic (i.e.,
nutrient-poor), due to their young age, high volume to surface area ratio, and proximity to
rock that is generally poor in organic carbon (exceptions include coal or lignite mines).
Some pit lakes may initially have high nitrate concentrations from explosives residue, or
in cases where cyanide-rich process water has been oxidized and disposed of in the pit.
Chemical and/or biological reactions may sequester nutrients in near-surface water and
store them in sediment at the bottom of the lake. Extremely low nutrient concentrations
will limit primary productivity by algae and aquatic plants, which can leave a lake
relatively barren with respect to higher forms of aquatic life.
2.4.2 Review of chemical processes
Geochemical processes taking place in mining pit lakes have been reviewed by Miller et
al. (1996), Geller et al. (1998), Shevenell et al. (1999), and Eary (1998, 1999), among
others. Figure 11 and Table 1 summarize some of the more important chemical
processes that are likely to occur. Many of these reactions are mediated by microbes
(algae and bacteria). The following sections provide additional details and discussion.
Figure 11. Summary of chemical processes occurring in mining pit lakes.
epilimnion
hypolimnion
Fe
3+
H
+
, Fe
2+
adsorption,
mineral dissolution,
subaqueous pyrite
oxidation
monimo-
limnion
pit sediment
O
2
diffusion
Fe
2+
→ HFO
evaporation
seasonal
overturn
leaching of
soluble salts
from weathered
bedrock
photochemical reactions
Fe-oxidizing
bacteria
water
table
landslides
precipitation
algae ← photosynthesis
gravitational
settling
←
bacterial Fe reduction
←
bacterial sulfate reduction ?
methanogenesis ?
← denitrification
groundwater
influx

19
Table 1. Summary of chemical processes occurring in pit lakes. The information is
arranged from top to bottom in the approximate order of increasing depth in the lake.
PROCESS IMPACTS REFERENCES
Shallow water
Evaporation Concentration of all solutes in proportion to
the fraction of water lost to vapor. May lead to
precipitation of gypsum, calcite, and other
sparingly soluble salts.
Levy et al.
(97); Eary (98);
Shevenell
(00a)
Photosynthesis by
algae, cyano-
bacteria, and plants
Increase in biomass which provides carbon
source for bacteria, animals, and other
decomposers. Increase in DO. Increase in pH
(for non-acidic lakes). Possible uptake of
trace metals by plants. Blockage of sunlight if
conditions are eutrophic.
Ledin and
Pederson (96);
Nixdorf et al.
(98a, 98b);
Kalin et al.
(01)
Photoreduction Photoreduction of Fe
3+
or hydrous ferric oxide
to Fe
2+
. Possible photoreduction of Mn oxides
to Mn
2+
. Rapid attenuation of visible light,
especially at short wavelengths. Catalyst for
breakdown of dissolved organic carbon.
Collienne (83);
Friese et al.
(02); Diez
Ercilla et al. (in
press)
Leaching of soluble
salts stored above
the water line
Increase in salinity and concentrations of trace
metals and metalloids.
Newbrough
and Gammons
(02); Maest et
al. (04)
Freezing Increase in salinity of water during ice
formation due to salt exclusion. May lead to
thermohaline convection below ice cover.
Dilution of surface water during spring thaw.
Gibson (99a,
99b)
Oxidation and
precipitation of
hydrous metal
oxides
Decrease in concentration of dissolved Fe and
Mn. Decrease in trace solutes that adsorb onto
hydrous metal oxide (see below). Increased
turbidity from suspended mineral particles.
Possible drop in pH.
Jonas (00);
Pellicori et al.
(05); Eary (99);
Dowling et al.
(04)
Adsorption onto
suspended mineral
particles or organic
matter
Decrease in concentration of heavy metals and
metalloids. Enhanced removal of As, Se at
low pH. Enhanced removal of Cd, Cu, Pb, Zn
at high pH. Important sink for phosphate.
Eary (99);
Smith (99);
Tempel et al.
(00)
Dissolution of
minerals exposed on
submerged mine
walls
If carbonate minerals are present, can be
important mechanism for buffering pH to
near-neutral conditions. If lake is acidic, may
see conversion of feldspar and other rock-
forming minerals to clay.
Eary (99);
Bowell and
Parshley (05);
Shevenell et al.
(99)
Sub-aqueous
oxidation of pyrite
by O
2
and/or
dissolved Fe
3+
Increase in concentration of dissolved heavy
metals and sulfate. Drop in pH. Drop in
dissolved oxygen and/or dissolved Fe
3+
concentration.
Madison et al.
(03); Pellicori
et al. (05)

20
Table 2. (Continued from previous page)
Deep water
Aerobic respiration
of organic carbon
Dissolved oxygen is depleted or completely
consumed.
Alvarez-
Cobelas (90)
Subaqueous
oxidation of pyrite
by dissolved Fe
3+
If water is acidic, high concentrations of Fe
3+
may persist at depth. Fe
3+
is a strong oxidant
of pyrite and other metal sulfides, resulting in
a drop in pH and release of Fe
2+
, sulfate, and
other metals.
Madison et al.
(03); Pellicori
et al. (05)
Microbial reduction
of Fe and Mn oxides
Increase in dissolved Fe
2+
and Mn
2+
concentration. Increase in pH. May be major
cause of high-salinity bottom water that can
induce meromixis. May release adsorbed
metals and nutrients to water column, such as
arsenic and phosphate.
Blodau et al.
(98); Wendt-
Potthoff et al.
(02); Sanchez-
Espana et al.
(07)
Microbial reduction
of nitrate
Reduction of dissolved nitrate (if present) to
N
2
and/or ammonia.
Helmer and
Labroue (93)
Microbial sulfate
reduction
Decrease in dissolved sulfate. Increases in
hydrogen sulfide (H
2
S or HS
-
), pH, and
bicarbonate alkalinity. Drastic decrease in
concentrations of metals (e.g., Cd, Cu, Fe, Ni,
Zn) that form insoluble metal sulfide
precipitates. Possible buildup of H
2
S (a
poisonous gas) to dangerous levels.
Blodau et al.
(98); Castro et
al. (99);
Harrington et
al. (04);
Fauville et al.
(04)
Methylation of Hg If present, inorganic Hg may be converted to
methyl-Hg, resulting in rapid bio-
accumulation in organisms.
Wolfe and
Norman (98);
Gray et al (03)
Methanogenesis May occur in pit lake sediment with a high
organic C to sulfate ratio. Increase in
concentrations of dissolved organic carbon,
CH
4
and H
2
.
Blodau et al.
(98);
Holowenko et
al. (00)
2.4.2.1 Evaporation.
Evaporation increases the salinity of a lake, especially terminal pit lakes in dry climates.
Eary (1998) gives a good discussion of predicted chemical pathways for pit lakes of
contrasting starting chemistry. As a general rule, lakes that fill with acidic, metal-rich
water will become more acidic and more metal-rich with evaporation, although mineral
saturation may provide an upper limit to the concentrations that a given dissolved solute
(e.g., Fe, Al, SO
4
) may attain. Lakes that fill with alkaline water rich in bicarbonate and
sulfate are likely to precipitate calcite and gypsum upon evaporation, and may end up
with quite a high pH (e.g., > 9). This increase in pH may increase the concentration of
certain dissolved contaminants, such as As and Al, whose solubilities increase at very
high pH. Although evaporation will be most pronounced in hot, dry climates (such as

21
Nevada or Australia), it can still be a significant process in climates that are cold and dry.
For example, Gammons et al. (2006b) estimated that the hypolimnion and epilimnion of
the Berkeley pit lake in Butte, Montana, were about 25% evaporated, based on trends in
the stable isotopic composition of the water column. Over long periods of time (e.g.,
100s of years) evaporation of surface water in terminal meromictic lakes may result in an
inversion in the density profile of the lake, thereby inducing overturn.
2.4.2.2 Photosynthesis. Most pit lakes, regardless of pH, contain planktonic algae and
cyano-bacteria that photosynthesize. Lakes with near-neutral pH may also have rooted
macrophytes (e.g., reeds or lily pads) along shorelines and in littoral areas. For aquatic
plants, the overall photosynthesis reactions can be written as follows:
CO
2
(aq) + H
2
O(l) → CH
2
O(s) + O
2
(10)
HCO
3
-
+ H
2
O(l) → CH
2
O(s) + O
2
+ OH
-
(11)
where CH
2
O(s) is an abbreviation for organic carbon. Although reaction (10) is more
familiar to most readers, many plants and microbes can switch to reaction (11) under
conditions of high pH when dissolved CO
2
concentrations are extremely low (Vymazal,
1995). In either case, photosynthesis by green plants and algae produces DO and usually
results in an increase in pH either by consumption of CO
2
(reaction 10), or production of
OH
-
(reaction 11). Alkaline lakes with very high productivity may see mid-summer pH
values in the 9 to 10 range (Alvarez-Cobelas et al., 1990), and may also exhibit diurnal
(day-night) patterns of higher pH and DO during the day and lower pH and DO during
the night. In contrast, photosynthesis has little or no influence on the pH of strongly
acidic lakes. This is because the H
+
concentration of low pH lakes is controlled by
reactions involving dissolved Fe, Al, and S species, and is less influenced by changes in
CO
2
concentration. Besides influencing the DO concentration and pH of the epilimnion,
photosynthesis is also important in fixing organic carbon which can provide food for
higher organisms. Algae are known to “leak” sugars and other substances that can be
used as organic substrate by bacteria and other decomposers (Vymazal, 1995).
The rate of photosynthesis in a lake is dependent on sunlight intensity and nutrient
availability (Wetzel, 2001). The depth to which light penetrates the water column can be
approximated by lowering a black and white secchi disk into the water and noting the
depth where the disk is no longer visible to the eye. Photosynthesis can proceed at a
significant rate under ice cover, particularly early or late in the winter if the ice is thin and
snow is scarce. For lakes at high latitudes or altitudes with thick ice and abundant
snowfall, very little sunlight will penetrate to the epilimnion, and therefore
photosynthesis will be severely limited in mid- or late-winter. A negative consequence is
that DO levels in the epilimnion may drop during winter if there are sources of biological
or chemical oxygen demand (e.g., Greenbank, 1945).
Essential nutrients for photosynthesis include nitrate (NO
3
-
) and phosphate (PO
4
3-
).
Algae uptake C, N, and P in the approximate ratio of 106:16:1 (in molar units), as shown
by the following equation (Redfield et al., 1963):

22
106CO
2
+ 16NO
3
-
+ HPO
4
2-
+ 122H
2
O + 18H
+
= C
106
H
263
O
110
N
16
P(algae) + 138O
2
(12)
Many pit lakes will have an excess of nitrate relative to phosphate compared to the ideal
“Redfield ratio”, due to inputs of nitrate from blasting residue or breakdown of cyanide.
However, this is not always the case. For example, the Berkeley pit lake of Butte,
Montana has PO
4
concentrations in the range of 0.1 to 1 mg/L as P (Jonas, 2000), but
little or no detectable nitrate. Concentrations of nutrients in pit lakes can be increased by
fertilization or addition of nutrient-rich waste water. Careful thought should be given to
any nutrification project, as over-application of fertilizer or waste water may lead to
eutrophic conditions that could have undesirable consequences, including growth of
nuisance algae or rooted plants, or severe depletions in DO in parts of the lake where
plant matter is decomposed.
2.4.2.3 Other photochemical reactions.
Besides providing energy for biological synthesis, a number of abiotic
oxidation/reduction reactions are catalyzed by sunlight. These include photooxidation of
dissolved organic carbon and photoreduction of dissolved or particulate forms of ferric
iron (Fe
3+
). Both of these processes attenuate visible light, and in extreme cases may
result in water that appears black at depths of a meter or two, even in the absence of any
turbidity. Although there are only a few studies documenting this phenomenon (Friese et
al., 2002; Sanchez-Espana et al., 2007; Diez Ercilla et al., in press), photoreduction of Fe
is probably a ubiquitous process in the near-surface water of mining lakes that are acidic
and iron-rich. Many studies have shown that photoreduction results in day-time increases
in Fe
2+
and total dissolved Fe in acidic rivers and streams (McKnight et al., 1988;
Gammons et al., 2005, 2008), as well as in natural lakes with low pH (Collienne, 1983)
or circumneutral pH (Emmenegger et al., 2001). During the night, Fe
2+
is re-oxidized to
Fe
3+
, and may precipitate as HFO or other ferric compounds such as schwertmannite or
jarosite. Although photoreduction of dissolved iron is faster than photoreduction of
particulate iron, the latter reaction can also occur. The soluble Fe
2+
produced by
photoreduction can then be utilized by algae as an essential nutrient (Emmenegger et al.,
2001) or may be re-oxidized to Fe
3+
by iron-oxidizing bacteria (IOB).
2.4.2.4 Leaching of soluble salts.
Open pit mining creates large areas of fractured and blasted rock that are freshly exposed
to the elements. This accelerates the rate of physical and chemical weathering. If the
rock contains sulfide minerals, then exposure to oxygen and water will result in acid
formation and leaching of metals from the rock (Nordstrom and Alpers, 1999). During
wet periods, the acid and metals released may be washed directly into the pit lake, or may
infiltrate into the subsurface along cracks to the water table, and thence to the lake via
groundwater inflow. In contrast, during extended dry periods much of the acid and
metals released from chemical weathering may accumulate on the outcrop surface as
efflorescent (i.e., water-soluble) salts. There are a very large number of secondary phases
that can form in this environment (e.g., see Alpers et al., 1994; Bowell and Parshley,
2005), but most of the common minerals are sulfates of rock-forming elements such as

23
Al, Ca, Fe, and Mg, and sulfates or other compounds containing trace metals such as Cu,
Pb and Zn. In low pH environments, some of these salts may also contain hydronium ion
(H
3
O
+
), which is one form of “stored acidity”. Later on, if the pit lake waters rise or
during the next major rain event, the soluble salts will dissolve and be flushed into the
lake, possibly leading to episodic water quality problems. The potential for poor quality
water to reach the shallow lake by this process can be quantified by performing
laboratory or field leaching tests (described below).
2.4.2.5 Precipitation of hydrous ferric oxide.
Many mining pit lakes contain elevated concentrations of dissolved Fe. Figure 12 is an
Eh-pH diagram that shows environmental conditions where Fe is soluble as ferric (Fe
3+
)
or ferrous (Fe
2+
) ions, and where it is insoluble and will precipitate as HFO (Fe(OH)
3
),
siderite (FeCO
3
), or pyrite (FeS
2
). Because most meromictic pit lakes have anoxic deep
waters, they often contain elevated concentrations of dissolved Fe
2+
, even at pH values
greater than 6 (see location “A” in Fig. 12). In such cases, Fe
2+
will be oxidized to HFO
or another ferric compound (such as schwertmannite, see Bigham and Nordstrom, 2000)
in the overlying oxygenated water column, as shown by the following reaction:
Fe
2+
+ ¼O
2
(aq) + 5/2H
2
O = Fe(OH)
3
(s) + 2H
+
(13)
This reaction is normally catalyzed by IOB, such as Acidithiobacillus ferrooxidans
(Nordstrom, 2003). Although this reaction is effective at decreasing the dissolved Fe
concentration of mine waters, it has two deleterious effects: 1) bacterial oxidation of Fe
2+
can deplete the water column of DO; and 2) the protons released in reaction (13) can
result in a drop in pH at shallow depths in the lake. Whether or not pH drops to
problematic levels depends on the balance between the rate of Fe-oxidation and the
presence or absence of alkalinity in the water. For example, a pH-neutral or alkaline lake
with high concentrations of bicarbonate ion (HCO
3
-
) can normally buffer the acid
generated by reaction (13) without much drop in pH (see path C in Fig. 12). In contrast,
if the shallow water has low TDS and low or zero alkalinity, then the protons released
during Fe oxidation and hydrolysis may well drop pH below 4 or, in some cases, below 3
(path B in Fig. 12).
Precipitation of HFO near the anoxic/oxic interface of mining pit lakes can lead to
scavenging of dissolved solutes that have a high affinity for adsorption onto the Fe-oxide
surfaces. Because of its very small grain size and irregular shape, freshly precipitated
HFO has a huge specific surface area, estimated to be as high as 250 to 600 m
2
per g of
solid (Langmuir, 1997). A large proportion of the unsatisfied bonds at the surface of
HFO particles wind up as potential sites for adsorption of solutes from the bulk water.
Different solutes have different affinities for adsorption onto HFO, and these affinities
are strongly pH-dependent. In general, HFO will selectively adsorb anions (negatively
charged solutes) at low pH, and cations (positively charged solutes) at near-neutral to
alkaline pH. The reason for this behavior is that the HFO surfaces behave in an
analogous fashion to a polyprotic acid. At low pH, the surfaces are protonated and carry
a net (+) charge which attracts negatively-charged anions. As pH increases, the surface
sites become deprotonated, develop a neutral or (-) charge, and attract cations. Examples

24
of anions that are strongly adsorbed onto HFO in low pH waters include arsenate
(HAsO
4
2-
or H
2
AsO
4
-
), sulfate (SO
4
2-
), selenate (SeO
4
2-
), phosphate (H
2
PO
4
-
or HPO
4
2-
)
and DOC. Although DOC is a mixture of molecules of different stoichiometry, many of
Figure 12. Eh-pH diagram showing the general conditions where Fe is soluble (unshaded
areas) and insoluble (shaded areas). The diagram was drawn for 10 mg/L Fe, 10 mg/L C,
and 100 mg/L S.
these are weak acids that lose their protons and carry a (-) charge at pH > 2, and therefore
adsorb strongly onto Fe-oxides (Tipping, 1981). As pH increases, anionic solutes desorb
from the HFO surfaces and are sequentially replaced by metal cations. As shown in
Figure 13, each metal cation has a characteristic pH where it will adsorb onto HFO, often
referred to as the adsorption edge. Because Pb
2+
and Cu
2+
have a higher chemical affinity
for HFO surface sites, they adsorb at lower pH than other solutes such as Zn
2+
or Ca
2+
.
In reality, the situation is more complex than is depicted in Figure 13, since different
solutes “compete” for available sorption sites. As well, it has been shown that some
solutes that are attracted to hydrous metal oxide surfaces, such as dissolved organic
carbon, can act as a “bridge” between the positively-charged HFO surface and the
positively charged metal cations in solution. This phenomenon is sometimes referred to
as “co-adsorption” (Sheals et al., 2003).
Adsorption of trace solutes onto HFO can be either good or bad. Clearly any process that
lowers the dissolved concentration of a toxic substance such as As or Pb is good. On the
flip side, sorption of phosphate and DOC may leave shallow water impoverished in
Eh, volts
1
0.8
0.6
0.4
0.2
0
-0.2
-0.4
pH
0 2 4 6 8 10
O
2
H
2
O
H
2
O
H
2
Fe(OH)
3
s
i
d
e
r
i
t
e
pyrite
Fe
2+
Fe
3+
Fe(OH)
2+
BC
A

25
nutrients, resulting in lower primary productivity by algae and aquatic plants which are
the base of the aquatic food chain. In either case, it is important to remember that
adsorption is a reversible process, and therefore ions will exchange freely as conditions in
the water column change (Drever, 1997).
Figure 13. Adsorption of selected cationic trace metals onto hydrous ferric oxide as a
function of pH. Diagram modified from Drever (1997).
2.4.2.6 Subaqueous water-rock interaction.
After the initial flush of secondary salts during filling of a pit lake, the rate of leaching of
minerals from submerged bedrock will diminish. However, water-rock interactions may
still play a role in modifying or buffering the chemistry of the lake. For example, if
limestone, dolomite, or other carbonate-rich rock types are present, then dissolution of
the carbonate minerals will add alkalinity and help maintain pH values > 6. The key
acid-buffering reaction can be written as follows:
CaCO
3
(s) + 2H
+
= Ca
2+
+ H
2
O + CO
2
(aq) (14)
Even if the host rocks are igneous, carbonate minerals may be present in veins or
disseminated through hydrothermally altered rock. This material can be an important
source of acid neutralization potential (ANP or NP) that will help maintain circum-
neutral or alkaline pH values.
Compared to carbonate minerals, most rock-forming silicate minerals are much slower to
react with water. However, some silicate minerals are more reactive than others. In
general, the rate of weathering of silicate minerals found in igneous rock is more or less
proportional to their position in “Bowen’s Reaction Series” (Fig. 14). Anhydrous
minerals such as olivine or Ca-rich plagioclase that crystallize from magmas at the
highest temperatures and pressures are furthest from equilibrium in the near-surface
weathering environment. If present on the submerged walls of a pit lake, such phases
will break down or transform to more stable minerals, such as clay, and will release silica
2 4 6 8 10
% adsorbed
20
0
40
60
80
100
pH
Cr
3+
Pb
2+
Cu
2+
Zn
2+
Cd
2+
Ca
2+

26
and metal cations (e.g., Ca
2+
, Mg
2+
, and Fe
2+
) to solution Example reactions for
anorthite (Ca-rich plagioclase) and forsterite (Mg-rich olivine) can be written as follows:
CaAl
2
Si
2
O
8
(s) + 2H
+
+ H
2
O = Al
2
Si
2
O
5
(OH)
4
(kaolinite) + Ca
2+
(15)
Mg
2
SiO
4
(s) + 4H
+
= 2Mg
2+
+ H
4
SiO
4
(aq) (16)
Because these reactions consume protons, the presence of minerals like Ca-rich
plagioclase, olivine, or pyroxene can add a significant amount of acid neutralization
potential to the wallrock. In contrast, minerals such as quartz, K-feldspar, and muscovite
that are near the bottom of Bowen’s Reaction Series are closer to equilibrium with
ambient conditions at the Earth’s surface, and therefore are extremely slow to react.
Following this train of thought, if follows that mafic or ultramafic rocks (e.g., basalt,
gabbro, amphibolite, peridotite) will have higher reactivity and acid neutralization
potential than felsic or silicic rocks (e.g., granite, rhyolite, sandstone, quartzite).
Figure 14. Bowen’s Reaction Series, showing the sequence of crystallization of common
silicate minerals from magma. Minerals near the top of the series are furthest from
equilibrium with the Earth’s surface and will chemically weather at a faster rate
.
2.4.2.7 Subaqueous pyrite oxidation.
It is commonly stated that the three essential ingredients for pyrite oxidation are pyrite,
oxygen, and water, and the overall reaction is often written as follows:
FeS
2
(s) + 7/2O
2
(aq) + H
2
O → Fe
2+
+ 2SO
4
2-
+ 2H
+
(17)
DO concentrations in lakes are typically < 10 mg/L, or approximately 0.3 x 10
-3
mol/L.
About 0.2 x 10
-3
mol/L of H
+
would be generated if all of this DO was used up by
reaction (17), enough protons to drop the pH of the water to ~ 3.5 to 4 assuming no
competing reactions. However, because diffusion rates of O
2
in water are extremely
slow, water below the wave zone that has lost its DO may not see oxic conditions again
until the next turnover event. This effectively shuts off the pyrite oxidation reaction with
respect to equation (17) for water in the hypolimnion or monimolimnion of stratified
lakes.
Ca-rich
Na-rich
olivine
pyroxene
amphibole
biotite
Increasing rate
of chemical
weathering
p
l
a
g
i
o
c
l
a
s
e
muscovite
K-feldspar
quartz
Order of
crystallization
from magma

27
Although O
2
is the most familiar chemical species that drives pyrite oxidation, Fe
3+
– if
present - is actually a much more aggressive oxidant than O
2
. The overall reaction for
pyrite oxidation by Fe
3+
can be written as follows (Nordstrom and Alpers, 1999):
FeS
2
(s) + 14Fe
3+
+ 8H
2
O → 15Fe
2+
+ 2SO
4
2-
+ 16H
+
(18)
Ferric iron is insoluble at pH > 4, but in strongly acidic waters (pH < 3) it can accumulate
to concentrations greater than 100 mg/L. Under these conditions, pyrite on submerged
mine walls may be rapidly oxidized by Fe
3+
, releasing a large quantity of protons (16
moles for each mole of pyrite reacted) and Fe
2+
. Although this reaction consumes Fe
3+
quickly, there is normally a large reservoir of Fe
3+
in acidic pit lakes, especially when one
considers secondary minerals (HFO, schwertmannite, jarosite) on mine walls and
suspended in the water column. If dissolved Fe
3+
concentrations diminish through pyrite
oxidation, these Fe-rich minerals can re-dissolve, releasing more Fe
3+
that can drive
reaction (18) to the right. In this way, pyrite oxidation can continue in the complete
absence of O
2
(Madison et al., 2003).
The only studies that have stressed the potential importance of subaqueous pyrite
oxidation in a mining pit lake are those of Madison et al. (2003) and Pellicori et al.
(2005), both of whom focused on the Berkeley pit lake of Butte, Montana. Madison et al.
(2003) concluded that millions of moles of protons are probably added to the deep pit
lake each year via reaction (18), and Pellicori et al. (2005) gave stable isotope data in
support of this hypothesis. Although subaqueous oxidation of pyrite by Fe
3+
is probably
a common (and usually overlooked) phenomenon in acidic lakes, this process is not
expected to be important in pit lakes with near-neutral or alkaline pH, mainly because the
solubility of Fe
3+
is so low at pH > 4.
2.4.2.8 Bacterial reduction of iron and sulfate
Most redox reactions taking place in deeper waters of mining pit lakes are catalyzed by
bacteria (Nordstrom, 2003). Heterotrophic bacteria utilize dissolved organic carbon
(DOC) as a source of electrons to reduce compounds such as dissolved oxygen, nitrate,
Mn or Fe oxides, or sulfate. Example balanced reactions are given below wherein the
source of organic carbon in each case is taken as acetate (CH
3
COO
-
), a common form of
DOC:
CH
3
COO
-
+ 2O
2
(aq) + H
+
→ 2CO
2
(aq) + 2H
2
O (19)
CH
3
COO
-
+ 8/5NO
3
-
+ 13/5H
+
→ 4/5N
2
(g) + 2CO
2
(aq) + 14/5H
2
O (20)
CH
3
COO
-
+ 4MnO
2
(s) + 9H
+
→ 4Mn
2+
+ 2CO
2
(aq) + 6H
2
O (21)
CH
3
COO
-
+ 8Fe(OH)
3
(s) + 17H
+
→ 8Fe
2+
+ 2CO
2
(aq) + 22H
2
O (22)
CH
3
COO
-
+ SO
4
2-
+ 3H
+
→ H
2
S + 2CO
2
(aq) + 2H
2
O (23)
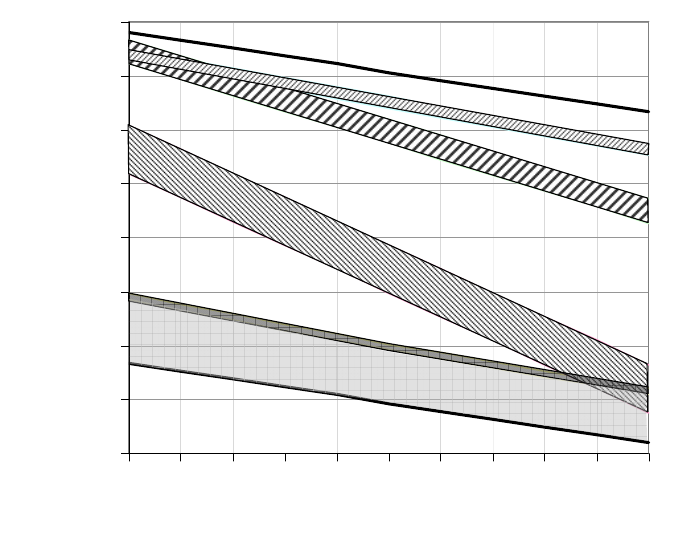
28
Under strongly reducing conditions where elevated levels of dissolved H
2
gas are present,
microbes may reduce DOC to form methane, a process that is referred to as
methanogenesis:
CH
3
COO
-
+ 4H
2
(aq) + H
+
→ 2CH
4
(aq) + 2H
2
O (24)
All of these reactions are thermodynamically favorable, and the energy gained by
catalyzing each redox reaction helps drive the microbes’ metabolism. In general, the
relative amount of energy to be gained from each of the above reactions decreases in the
approximate order given. As a result, aerobic bacteria who make use of reaction 19 will
typically dominate as long as DO is present. When all DO is consumed, then anaerobic
bacteria will reduce nitrate next, then Mn, then Fe, and lastly sulfate. In reality, most of
these reactions occur over a range in redox conditions, and the Eh (i.e., oxidation state) at
which a given reaction begins is also strongly pH-dependent, as illustrated in Figure 15.
For example, the Eh ranges corresponding to Fe reduction and sulfate reduction overlap
at high pH, but are widely separated at lower pH.
Figure 15. Diagram showing the approximate Eh and pH conditions of selected bacterial
reactions that may be important in mining pit lakes. All data used to draw this diagram
were taken from Drever (1997). Fe and Mn reduction lines assume equilibrium with
ferrihydrite and birnessite, respectively. Upper and lower limits for Fe and Mn reduction
were taken at 1 µmol/L and 1 mmol/L dissolved Fe
2+
and Mn
2+
, respectively. Upper and
lower limits for sulfate reduction were taken at SO
4
2-
/(H
2
S+HS
-
) ratios of 1000 and 1,
respectively. Upper and lower limits for nitrate reduction were taken at NO
3
-
= 0.1
mmol/L, and P(N
2
) = 0.001 and 1 bar, respectively.
-0.6
-0.4
-0.2
0
0.2
0.4
0.6
0.8
1
4.5 5 5.5 6 6.5 7 7.5 8 8.5 9 9.5
pH
Eh, V
F
e
r
e
d
u
c
t
i
o
n
m
e
t
h
a
n
o
g
e
n
e
s
i
s
M
n
r
e
d
u
c
t
i
o
n
d
e
n
i
t
r
i
f
i
c
a
t
i
o
n
s
u
l
f
a
t
e
r
e
d
u
c
t
i
o
n
l
o
w
e
r
s
t
a
b
i
l
i
t
y
l
i
m
i
t
o
f
w
a
t
e
r
u
p
p
e
r
s
t
a
b
i
l
i
t
y
l
i
m
i
t
o
f
w
a
t
e
r
29
Examination of reactions 19 through 23 shows that bacterial oxidation of DOC typically
consumes protons (thereby raising pH) and produces CO
2
. The increase in pH
accompanying reactions such as iron or sulfate reduction can be significant, and may
improve the water quality of deeper waters in acidic pit lakes that are iron- or sulfate-rich.
This is particularly true of sulfate reduction, as the H
2
S that is produced by bacteria will
combine with dissolved heavy metals to form insoluble metal sulfides, as shown in the
following generic reactions:
Me
2+
+ H
2
S = MeS(s) + 2H
+
(25)
Me
2+
+ HS
-
= MeS(s) + H
+
(26)
where Me
2+
refers to a divalent heavy metal such as Cd
2+
, Cu
2+
, Fe
2+
, Ni
2+
, Pb
2+
, or Zn
2+
.
Reaction (25) applies at pH ≤ 7, whereas reaction (26) applies at higher pH. Metal
sulfides formed by either reaction will slowly settle by gravity to the bottom of the lake
and will accumulate in sediment, where they may undergo diagenetic transformations that
increase their particle size and crystallinity. Such a process drives the slow conversion of
nanocrystalline FeS(s) to micron- or millimeter-sized grains of pyrite (FeS
2
).
Although bacterial iron and sulfate reduction may raise the pH of a lake, they may also
have undesirable consequences. For example, reactions (21) and (22) will increase the
dissolved concentrations of Fe and Mn, which may then diffuse or convect (during lake
overturn) upwards to shallower levels where re-oxidation occurs, resulting in re-
precipitation of hydrous Fe and Mn oxides and release of protons. Reductive dissolution
of Fe and Mn oxides may release trace metals or metalloids that were adsorbed onto the
solid particles (Hongve, 1997). This is particularly true of arsenic, phosphate, and DOC,
all of which have a strong affinity for sorption onto HFO. In some lakes, bacterial Fe
reduction can be an important factor in increasing the TDS of bottom water, thereby
promoting meromixis. This may be good or bad, depending on the intended end-use of
the pit lake. Lakes with high microbial activity may also see dramatic increases in the
partial pressure of CO
2
and possibly H
2
S, especially in deep waters near the sediment
interface. In extreme cases, high concentrations of dissolved gas may be dangerous in the
event of a top-to-bottom turnover (see Section 2.3.4).
2.5 BIOLOGY OF PIT LAKES
Understanding the biology of mine pit lakes, including the invasion of pioneer and
successional species, is vital when considering effective reclamation strategies. Although
the physical, chemical and limnological characteristics of pit lakes are relatively well
understood, there is little information available regarding the biological characteristics of
such systems. Since pit lakes are often contaminated with heavy metals and/or low pH
(Nixdorf et al., 1998a, 1998b; Wollmann et al., 2000), much of the research on the
biology of pit lakes has focused on trying to understand the trophic organization in such
inhospitable environments (Klapper and Schultze, 1995). Information on the ecology of
pit lakes that have chemical and physical properties similar to other natural lakes in

30
similar areas is still quite scarce. Furthermore, most research on pit lake colonization and
habitat use have focused on bacteria, phytoplankton, zooplankton, and to a lesser degree
invertebrates (e.g., Nixdorf et al., 1998a, 1998b; Steinberg et al., 1998). Information on
the establishment of macrophytes in and around these water bodies, and the use of habitat
in such systems by wildlife and fishes is much less available.
The species diversity of a mine pit lake will be highly influenced by the specific natural
biological diversity that persists in other waterbodies in the vicinity. Biocenoses in pit
lakes, additionally, will be dependant on various and complex factors including how the
lake was filled and the initial physical and chemical conditions of the water body
(Tandonleke et al., 2000; BHP 2005). In many cases, immediately after pit lake
formation, these habitat conditions (e.g., light availability, metal concentrations, nutrient
levels, and pH) are quite stressful resulting in biological diversity that is low, or even
absent, especially at higher trophic levels (Roch et al., 1985; Klapper and Schultz, 1995;
Nixdorf et al., 1998a, 1998b). Additionally, littoral zones and associated habitat are often
scarce or absent due to the steep-sided contour of these pits (Kalin et al., 2001). The lack
of this productive habitat will limit the establishment of aquatic vegetation and therefore
micro- and macro-invertebrates, fishes, and other wildlife. Finally, these water bodies are
often deep, with small surface area to depth ratios often resulting in meromixis and
permanent anoxia at depth (Castro and Moore, 2000; BHP, 2005). Because of these
characteristics, pit lakes tend to have limited biological activity and chemical interactions
dominate (Castendyk and Webster-Brown, 2006). Not surprisingly, the biological
community of a newly formed pit lake differs markedly from that of natural lakes in the
same area (BHP, 2005), with diversity and abundance being much lower in the former
(Klapper and Schultze, 1995). Since many of these characteristics will naturally change
with time after lake formation, the biological diversity will be different depending on the
age of the water body, with more taxa being present in older lakes (Lipsey and Malcolm,
1981).
2.5.1. Bacteria
Initially, in newly formed pit lakes some bacteria will exist (Klapper and Schultze, 1995),
but taxa richness and abundance will be dependant on lake-specific habitat characteristics
such as those listed above. If conditions are unfavourable, which is often the case when
pits are filled naturally with ground and surface water, the first colonists will be micro-
organisms that can tolerate such conditions (Steinberg et al., 1998; Wollmann et al.,
2000). These, in turn, could be abundant in certain cases due to reduced competition
(Klapper and Schultze 1995). For example, many studies document the presence of acidic
tolerant species of bacteria (e.g., Ochromonas spp., Acidithiobacillus spp.) (Klapper and
Schultze, 1995; Wollmann et al., 2000) when pH is quite low (pH < 3), a characteristic
often typical of pit lakes (Castro and Moore, 2000). As these acidic lakes stabilize and
water chemistry evolves towards a steady state (e.g., pH increases from acidic to neutral),
the community structure will change and the number of species can be expected to
increase (Kalin et al., 2001; Wollmann et al., 2000). Often mine pits are manually filled
by force flooding the pit with nearby water bodies to create the lake. If this is the case,
then initial bacterial and microbiota communities can be expected to be similar to the

31
contributing water body, however, usually with a fewer number of total species (eg.,
Tones, 1982). Additionally, in these manually filled waterbodies, the number of species
will be expected to increase with time since filling (Lipsey and Malcolm, 1981; Kalin et
al., 2001). In these systems, depending on the species, bacteria can cause acidification,
reduce DO levels, contribute to the cycling of metals and organic decomposition which
may in turn improve water quality (BHP, 2005), all important roles in aquatic
ecosystems.
2.5.2. Plankton
Phytoplankton and zooplankton communities may dominate the biota of pit lakes,
depending again on the water quality variables. Overall, plankton abundance is expected
to be low in acidic pit lakes or lakes contaminated with heavy metals (e.g., Zn and Cu).
Previous studies have shown that water chemistry is the main determinant of species
composition and diversity, but nutrient levels were the main determinant of biomass
(Nixdorf et al., 1998a,b; Lessmann and Nixdorf, 2000; Kalin et al., 2001). Additionally,
algae biomass can be expected to increase linearly with an increase in chlorophyll a
concentrations in the waterbody (Brenner et al., 1987). Regarding both phytoplankton
and zooplankton, marked differences in species composition is noticed with differences
in pH (Klapper and Schultz, 1995; Kalin et al., 2001). Although some acid tolerant
species may become established in pit lakes, as might be expected, these are often low in
biological diversity and total biomass (Wollann et al., 2000). Nixdorf et al. (1998a,b)
found species of Ochromonas and Chlamydomonas were dominant in acidic pit lakes of
Germany and more diverse algal assemblages with diatoms and cryptophytes persisted in
lakes with moderately acidic or alkaline conditions. Similarly, Klapper and Schultze
(1995) found that pioneer phytoplankton communities in acidic lakes were similar to
those in acidic bog water bodies in the same area of eastern Germany. Furthermore, if
newly formed pit lakes are characterized by extremely acidic conditions due to a lack of
bicarbonate, all algae groups of the Scenedesmus-type of photosynthesis are missing
(Klapper and Schultz, 1995) as they require this inorganic compound in order to exist.
Heavy metal contamination may also hinder zooplankton and phytoplankton diversity
and abundance in lakes as shown by Roch et al. (1985). In this study, it was found that in
Buttle Lake, Vancouver Island, diversity of planktonic species declined as a result of
nearby mining operations that have increased Zn and Cu concentrations in the waterbody.
They also found that some metal sensitive forms had disappeared altogether.
Zooplankton and phytoplankton density and diversity is also a function of the elapsed
time since lake creation. Brenner et al. (1987) studied phytoplankton and zooplankton
assemblages in 60 surface mine lakes in Pennsylvania and found that virtually all lakes
supported diverse aquatic plankton communities. The authors found, with respect to
phytoplankton, that the types present varied with the age of the lake. For example,
Pryophyta were more prevalent in younger lakes (< 10 years) and Chlorophyta and
Chrysophyta being more abundant in older lakes (20-30 years). Furthermore, they found
phytoplankton were not evenly distributed through the water columns of these pit lakes.
Pryophyta were more numerous at lower depths, whereas Cyanophyta and diatoms

32
prevailed at shallower depths. In the same study, 42 taxa of zooplankton were identified
and diversity of these organisms was found to correlate positively with the age of the lake
(Brenner et al., 1987).
If newly formed pit lakes are not acidic and have properties similar to other lakes in the
area then phytoplankton and zooplankton communities will initially first be colonized by
pioneer species that exist in the area, and will follow successional stages that are typical
in other lakes of the area (Tones, 1982). In some cases plankton establishment in pit lake
water bodies may also be mediated through the transfer of these species by waterfowl
(Arauzo et al., 1996). If pit lakes are created via flooding with surface water, then the
initial phytoplankton and zooplankton communities will be very similar to those of the
source water body (Kalin et al., 2001). Although usually not as biologically diverse as the
contributing water body (Tones 1982), the number of taxa should increase with time
(Lipsey and Malcolm, 1981). An open pit that is rapidly filled with water upon closure is
apt to have very high turbidity and poor light penetration. With time, suspended
sediment will settle to the bottom of the lake, which may allow the establishment of
phytoplankton communities (Kalin et al., 2001). Additionally, there may be diel or
seasonal changes in phytoplankton and zooplankton community structure (Armengol and
Miracle, 2000; Kalin et al., 2001).
2.5.3 Macrophytes
Literature pertaining to macrophytes is even scarcer than for the micro-biota. Pietsch
(1993) distinguished four stages of development of pit lakes: a macrophyte-free initial
stage (I), an early stage (II), a transition stage (III) and an old-age stage (IV) (cf. Klapper
and Schultz, 1995). The establishment of macrophyte communities in pit lakes will
depend on several factors including geographic location of the lake, stage of
development, water quality characteristics and physical lake characteristics. In the
absence of planting, and with favorable conditions with respect to the variables
mentioned above, the first aquatic macrophytes to colonize the lake will be pioneer plant
species native to the area. This will of course depend on the geographic location, but for
instance typical aquatic macrophyte pioneer species may include pondweed
(Potamogeton spp.) cattails (Typha spp), bulrushes (Scirpus spp) and sedges (Carex spp).
Tones (1982), assessing the Gunnar uranium mine in Northern Saskatchewan, found that
macrophytes were restricted to locations where haul roads entered the pit. Despite their
aerially restricted coverage, the aquatic vegetation was nonetheless fairly typical of the
area. This highlights the importance of mine reclamation in the form of landscaping or
backfilling to create littoral zones where aquatic vegetation can establish. Sumer et al.
(1995) found that lake morphometry and water levels had a bigger influence on
macrophyte abundance than did increasing nutrient levels in East Pit Lake, Alberta (see
section 5.3). This lake is relatively shallow (mean depth = 3.3m, Sumer et al., 1995)
indicating that littoral habitat is likely quite extensive.

33
2.5.4. Invertebrates
The presence of invertebrates in pit lakes depends on several characteristics, but the
establishment of littoral habitat with aquatic vegetation is crucial. If productive littoral
zones are established, either naturally over time, or created during the reclamation
process, invertebrates may be quite common. For instance, Sumer et al. (1995) found
diverse aquatic invertebrate assemblages in East Pit Lake, Alberta, a waterbody created
as part of a coal mine reclamation project. Tones (1982) found 22 taxa of invertebrates,
dominated by leeches, in the Gunnar uranium mine pit lake, most abundant in littoral
habitat. This pit was filled with water from a nearby lake, therefore pits filled passively
may exhibit quite different community structure. In deeper parts of the lake, invertebrate
numbers may be low, or they may be absent altogether (Tones, 1982). The persistence of
meromixis and anoxic conditions of bottom waters, combined with the steep-walled
nature of pit lakes, can result in the complete absence of a benthic community (BHP,
2005). Pelagic invertebrate communities can also be limited or absent due to reduced
nutrient availability and/or inhospitable conditions caused by low pH or high toxicity
caused by leaching of metals into the system (BHP 2005). Low pH may be a limiting
factor for many invertebrates, but some acid tolerant species (e.g., some species of
corixids) may nonetheless establish (Wollmann et al., 2000). Species richness will
typically be low in acidic lakes (Wollman et al., 2000), and pioneer species will need to
be able to tolerate harsh environmental conditions (Kalin et al., 2001).
2.5.5. Fish
Fish can come to inhabit pit lakes two ways: (1) introduction by flooding with source
water that contains fish or (2) deliberate stocking effort. Several examples exist in the
literature and both scenarios are discussed in further detail in section 4.2.5. If conditions
are favourable for fish survival, regardless of the method used for introduction, fish can
be abundant in pit lakes. Each fish species can tolerate a specific range of temperature
before being stressed. In Canada, freshwater fish species typically fall into two
categories: (1) coldwater fish species that prefer temperatures between 10-18
o
C, and (2)
coolwater fish species preferring temperatures ranging from 18-26
o
C (Nelson and Paetz,
1992). Fishes of northern Canada are represented by both groups of fish although the
majority would typically fall into the former category, including species such as lake
trout (Salvelinus namaycush), arctic char (Salvelinus alpinus) and Arctic grayling
(Thymallus arcticus). Thermal tolerance of a species is an important consideration when
deciding which species to introduce if supplementation is used to establish fish
communities in pit lakes.
Fishes also require waterbodies with an adequate supply of DO. Fishes will vary in their
DO requirements, depending on their stage of life history, weight, activity level, water
temperature, and feeding habits (Moyle and Cech, 2003). Since temperature and
biological processes directly influence DO concentration, there will be DO level
variations throughout the water column of the lake and levels will change depending on
the time of year or even time of day. Because of this, fishes may be forced to actively
move throughout the water column or to different parts of the lake to meet their DO
requirements. Coldwater fishes require relatively high DO concentrations in comparison

34
to coolwater fish species (Nelson and Paetz, 1992), and mortality in these groups of
fishes can occur if DO concentration falls below 2.0 mg/L (Doudoroff and Shumway,
1970). This may become an important consideration in northern pit lakes as they can be
isolated from atmospheric oxygen by ice cover for a large portion of the year.
Fishes also require a wide array of different habitats to complete their life cycle which
will vary depending on the species, time of year, life history stage and sex. Fish require,
directly and indirectly, very specific habitat for spawning and breeding, feeding, rearing,
habitat for cover and protection and habitat that will allow for the migration of fish
between these areas of use. In lakes, this will include areas such as gravel beds or rocky
shoals, and structures such as boulders, collections of woody debris, and undercut banks.
For example, juvenile fish will typically require areas that will provide sufficient cover
and therefore protection from predators whereas spawning adults may require rocky
shorelines or aquatic vegetation depending on the species. Since newly created pit lakes
are usually devoid of such habitat, it is important to incorporate reclamation practices that
include the creation and maintenance of fish habitat. For example, the creation of
underwater structures (logs, boulder clusters or rock piles) for cover or shoals for
spawning will be important for newly formed pit lakes. Additionally, since aquatic
vegetation is one of the most important factors for habitat selection (Pratt and
Smokorowski, 2003) the establishment and maintenance of littoral zones which include a
diverse array of aquatic macrophytes will be important for fish survival in pit lakes. Such
areas will undoubtedly provide more food and cover especially for small or juvenile
fishes.
2.5.5.1. Metal toxicity and fish.
Relative to uncontaminated, natural lakes in the same area, newly created pit lakes often
have elevated levels of metal concentrations and there is an enormous body of literature
on the negative effects of metals on the health of aquatic life (see Kelley, 1988, for a
comprehensive summary). For fishes, metal toxicity is often quantified by the so-called
“LC
50
” - the lethal concentration of a particular metal that would kill 50% of a batch of
organisms during a specified time period, usually 48 to 96 hours. As discussed by
Kelley (1999), the LC
50
varies from species to species and from metal to metal, and also
is a function of the bulk characteristics of the water, such as its salinity, hardness, and pH.
It is generally agreed that heavy elements are most toxic in their simple ionic forms, e.g.,
Al
3+
, Cu
2+
, Pb
2+
, Zn
2+
, and these “free ions” are most stable at low pH. Complexation of
the metal with a strong ligand, such as dissolved organic carbon, can significantly reduce
the bioavailability of the metal of concern (Santore et al., 2001). The presence or absence
of competing ions in solution is also a major factor determining metal toxicity. High
concentrations of Ca
2+
and Mg
2+
ions, in particular, are known to reduce the LD
50
for
most divalent heavy metals. This is why regulatory standards for protection of aquatic
life for many heavy metals (including Cd, Cu, Ni, Pb and Zn) are often not reported as
single values, but rather are a non-linear function of the water hardness (i.e., the
combined Ca
2+
+ Mg
2+
concentration of the water). It is also known that the presence of
multiple environmental stresses (e.g., the presence of both Cu and Zn in a water) can be
either additive, synergistic, or antagonistic. If additive, this means that the combined
effect is essentially the sum of the individual effects. If synergistic or antagonistic, this

35
means that the combined effect is greater than or less than the individual effects,
respectively. Kelley (1999) points out that very little is known about stress due to co-
exposure to different metals in aquatic ecosystems, especially when other forms of
environmental stress – such as extremes in temperature, dissolved oxygen, turbidity, or
pH – are involved.
Figure 16 (from Kelley, 1999) lists ranges in reported values of LC
50
for fishes based on
exposure to Ni, Cu, Zn and Pb. The large range in LC
50
for each metal is partly a
reflection of inter-species variation (e.g., note how salmonid species are more sensitive to
Cu than non-salmonid species), and partly a reflection of changes in the laboratory
conditions of each study, such as the duration of exposure, and also the pH and hardness
of the water used in the study. Nonetheless, it is clear from Figure 16 that the presence of
trace amounts (< 1 mg/L) of Cu, Pb, and Zn can be lethal to fishes. Much lower
concentrations of each metal would be expected to have a deleterious effect on overall
fish survival, such as lower reproduction rates or decreased ability to avoid predators.
Figure 16. Ranges in published data for LC
50
concentrations of selected heavy metals to
fishes (from Kelley, 1999).
2.5.6. Waterfowl
The literature on pit lake use by other forms of wildlife is quite scarce, and virtually all
studies to date have focused on habitat use of gravel pit lake systems by waterfowl. In the
Great Linford gravel pit, England, Phylips (1992) showed that waterfowl were common
inhabitants of the lake. In the newly flooded lake dabbling ducks dominated but as the
lake aged, macrophyte-feeding waterfowl became more common. In six gravel pit lakes
of France, Santoul et al. (2004) recorded 39 species of waterfowl. In this study, the
presence of paths also reduced abundance and diversity owing to human disturbance,
which is certainly an important factor in habitat selection. Therefore pit lakes where there
is an abundance of recreational use would also deter waterfowl from inhabiting the lake.
The most important factor influencing waterfowl use in pit lakes, however, is likely food
0.001 0.01 0.1
1.0 10
100
Metal in water (mg/L)
Pb
Zn
Cu
Ni
Non-salmonid
Salmonid
36
availability, which undoubtedly relates to the quantity and quality of vegetation found in
the water body (Phylips, 1992; Santoul et al., 2004). This may relate to the age of the
lake, water quality and physical characteristics of the waterbody. Additionally, other
factors such as competition for breeding locations and food will influence abundance and
diversity in these systems.
2.6. UNIQUE ASPECTS OF PIT LAKES IN NORTHERN ENVIRONMENTS
Pit lakes in northern areas such as the Northwest Territories of Canada may share certain
characteristics in common with lakes at lower latitude, but at the same time are likely to
have profound differences. The hypolimnion or monimolimnion of deep lakes in
temperate climates are cold year-round, with temperatures near or slightly above 4°C, the
maximum density of water. Stratified lakes in northern regions should have similar
temperatures for their deeper waters. Thus, many of the processes that take place below
the metalimnion of stratified lakes should be similar regardless of the latitude of the lake.
As an example, the limnology and geochemistry of the Udden pit lake in northern
Sweden (Ramstedt et al., 2003) is very similar to pit lakes with similar geology in more
temperate environments. On the other hand, some large differences are expected in
northern climates with extremely cold temperatures, especially with regards to ice
formation, permafrost, and biology of the epilimnion and surrounding terrestrial habitat.
Two key factors that have an impact on the limnology of northern lakes include low
average temperatures and very large, seasonal variations in solar insolation. At the Ekati
Diamond Mine located 300 km north of Yellowknife, NT, Canada, temperatures range
between -40
o
and +25
o
C with only four months having mean temperatures above freezing
(BHP, 1997). As a result, snow dominates the landscape for the majority of the year and
lakes are ice-covered more than they are ice-free (e.g., Pienitz et al., 1997a, b; Rikiishi et
al., 2004). The thickness of the ice cover depends on many factors, but can exceed 2 m
(Duguay et al., 2003). At some extreme northern locations, ice-cover can persist year
round (Doran et al., 1996). As a comparison, in Minnesota where there are more than
4000 abandoned quarry pits and over 200 abandoned iron ore pits, ice cover usually
persists for less than 5 months, from December to April (Axler et al., 1998).
In addition to extensive ice cover, northern regions are subject to large variations in light
availability, from near-total darkness during the winter to continuous sunlight during the
summer months. The Diavik Mine near Lac de Gras, NWT experiences two hours of
daylight during midwinter and 22 hours of sunlight during midsummer (Diavik Diamond
Mine Incorporated, 2007). Further north, at Colour Lake on Axel Heiberg Island, in
Nunavut, there are four months of darkness in the winter and four months of continuous
sunlight in the summer (Doran et al., 1996). The amount of solar radiation reaching the
surface is also influenced by cloud cover and cloud opacity (Young et al., 1995).
The combination of extensive ice cover and variable solar insolation has several possible
ramifications to pit lake dynamics, including the following:
-
extreme seasonal variation in primary productivity
-
seasonal variation in other photochemical reactions (such as Fe photoreduction)
37
-
increased risk of oxygen depletion during winter
-
thermohaline convection
-
increased likelihood of meromixis
Ice decreases light penetration to the underlying water column, and this can be further
exacerbated by the presence snow cover (Roulet and Adams, 1984; Duguay et al., 2003).
This means that photosynthesis (and other photochemical reactions, such as Fe
photoreduction, see Section 2.4.2.3) will be greatly diminished during periods of the year
with thick ice cover, especially during mid-winter months when the number of hours of
sunlight are at a minimum. In contrast, the long days of summer are conducive to much
higher rates of photosynthesis, provided that sufficient nutrients are available. Most large
lakes in northern Canada (e.g., Great Slave, Great Bear) are highly oligotrophic, with low
crops of planktonic or benthic algae and relatively sparse fish populations (Northcote and
Larkin, 1963; Livingstone, 1963). Barring an influx of nutrients from a man-made
source, similar oligotrophic conditions would be expected for mining pit lakes in the far
north. If so, primary production would most likely be nutrient-limited in the summer,
and light-limited in the winter. Interestingly, there is some evidence that primary
production in arctic lakes has been on the rise in the past century, most likely due to
global warming trends (Michelutti et al., 2005).
Once a lake is covered with ice, it is no longer possible for oxygen to freely diffuse into
the water column from air. If photosynthesis is diminished by ice cover and/or dim
sunlight, then there is also very little in situ production of oxygen. Given these
conditions, any biological oxygen demand (BOD, e.g., from bacteria or other respiring
organisms) or chemical oxygen demand (COD, e.g., from oxidation of pyrite or dissolved
Fe
2+
) could result in a reduction in the amount of DO in the water column. In extreme
cases (so-called “hypoxic” conditions), DO levels can fall below thresholds for fish
survival, a phenomenon known as a “fish winterkill” (Greenbank, 1945; Nickum, 1970;
Magnuson et al., 1989). Mortality for cold water fish species, such as those found in
most northern regions, may occur if DO concentrations fall below 2.0 mg/L (Doudoroff
and Shumway, 1970), and fishes inhabiting lakes with long-lasting ice cover are more
vulnerable to such hypoxic conditions (Evans, 2005). Alternatively, if the ice and snow
cover has a high transparency then photosynthesis can continue, albeit at a reduced rate
due to the low angle of the sun. Fish winterkills are a phenomenon most often associated
with shallow, eutrophic lakes as opposed to deep, oligotrophic lakes (Nickum, 1970).
However, the fact that pit lakes often have higher COD (e.g., from oxidation of dissolved
Fe
2+
or pyrite) means that they may be more susceptible to winterkill than natural lakes
with similar morphometry and climate.
As discussed in Section 2.3.5, formation of ice results in exclusion of dissolved solids,
which can then form brine streamers that sink by gravity through the water column. The
end result of this process (i.e., thermohaline convection) is an increase in the salinity of
deeper water in the lake. Later in the year, melting of the ice cover results in a low-
salinity, low-density layer on the top of the lake. Because of these two factors (ice
formation and ice melting), it is very possible that deep lakes in northern climates will
become density-stratified. The likelihood of meromixis is further increased if deeper
portions of the lake receive salty groundwater. According to one recent study (Kuchling
38
et al., 2000), the salinity of groundwater near the Diavik diamond mine in the NWT
increases with depth, attaining an average of ~ 1000 mg/L at 500 m below surface, and ~
6400 mg/L at 1000 m below surface. Highly saline groundwater is also common beneath
permafrost in the diamond fields of northern Russia (Borisov et al., 1995; Alexeev and
Alexeeva, 2003).
Associated with the cold climate, pit lakes in northern environments must also contend
with permafrost conditions. Permafrost is defined as ground (soil or rock and included ice
and organic material) that remains at or below 0°C for at least two consecutive years
(NRC Canada, 1988). Approximately half of Canada, and virtually all of northern
Canada, has permanently frozen ground (Heginbottom et al., 1995). Overlying the
permafrost is the active layer, a layer of substrate of varying depth depending on regional
conditions that thaws annually (NRC Canada, 1988). Permafrost is typically absent
beneath large lakes and rivers, even in northern climates (Mackay, 1997). However,
when a natural lake is drained, such as may happen when a diamond deposit is mined,
permafrost will build downwards from the newly exposed lake bottom and upwards from
the depth where permafrost starts until the two points of freezing merge (Mackay and
Burn, 2002). In the event that the open pit is later flooded, conduction of heat from the
water column may cause the newly formed permafrost to re-thaw. Besides these local
effects, permafrost can have substantial impacts on the regional flow of groundwater and
surface water, and can also lead to terrain instability with associated geotechnical
problems (Environment Canada, 2004). Permafrost must be taken into account for
accurate predictions of runoff and groundwater seepage and how these relate to filling
rates of pit lakes upon mine closure. The presence of frozen ground is also critical in the
design of treatment systems for contaminated drainage (Environment Canada 2004).
Figure 17 shows one possible scenario that could result when an open pit mine in
permafrost terrain is subsequently flooded. Besides the fact that the excavation opens up
a direct connection between the lake and the adjacent, sub-permafrost groundwater,
flooding of the mine void could result in thawing of the pre-mining permafrost zone
along the lake margins. Although each mine would need to be evaluated on a case-by-
case basis, it seems logical to assume that melting of permafrost on any steep slope would
increase the likelihood of slope failure (i.e., landslides). Other negative consequences
may include release of biogenic gas, such as methane or H
2
, either through enhanced
microbial activity due to the thawing conditions, or from thermal decomposition of
methane clathrate solids.

39
Figure 17. Schematic diagram of a pit lake in a permafrost setting. Conduction of heat
from the deep lake may gradually melt back the permafrost layer, resulting in decreased
slope stability and slope failure. Lakes that penetrate the permafrost layer may also serve
as conduits for deep groundwater recharge or discharge, depending on local
hydrogeological conditions. (The sketch depicts groundwater discharge).
The number of plant and animal species endemic to northern environments is low in
comparison to more southerly locations, and this is another factor to take into
consideration. For example, in the Northwest Territories and Nunavut combined, 46
species of fish have been identified that inhabit freshwater at least periodically during
their life cycle (Evans et al., 2002). In comparison, Minnesota, a state a fraction of the
size of Canada’s northern territories, harbors 160 species of freshwater fish (Hatch et al.,
2003), many of which reside in decommissioned pit lakes. This means that there are
fewer choices of candidate fish species in northern climates whenever decisions need to
be made regarding stocking or other efforts to establish a functional fishery. The same
observation applies for other types of biota, such as vascular plants. In the continental
Northwest Territories, Porsild and Cody (1980) describe 1113 plant species, whereas
Minnesota in comparison has nearly 2400 species (Ownbey and Morley, 1991) inhabiting
a much smaller area. The few number of species described at northern locales is
undoubtedly due to a combination of cold mean annual temperatures, and subsequent
short growing seasons and permafrost laden ground which makes it difficult for plants to
establish. Since plant growth is difficult in these northern environments, the vegetation in
such areas is typically composed of smaller trees in comparison to their southern
counterparts, and dwarf shrubs, graminoids, herbs, lichens and mosses, which all grow
relatively close to the ground (Porsild and Cody, 1980). This will be important for
reclamation considerations since the successful establishment of riparian and aquatic
vegetation will be integral for establishing and sustaining natural diversity in mines that
have been reclaimed into lakes. Furthermore, success in establishing vegetation in such
reclamation situations depends on proper plant species selection (Buttleman, 1992) which
is unquestionably limited in northern environments.
permafrost
permafrost
active layer
active layer
potential slope
failure
sub-permafrost
groundwater
permafrost retreat

40
3. PIT LAKE MODELING AND PREDICTION
There are many methods that have been proposed to predict the future water quality
characteristics of mining pit lakes. This section will first give the reader some idea of the
types of field or laboratory procedures that can be used to predict pit lake water quality.
This will be followed by a short review of computer models, with specific reference to
some of the more popular programs that are freely available from the web. What follows
is not meant to be an exhaustive review of the literature, as this is outside of the scope of
the document.
3.1 EXPERIMENTAL PROCEDURES
3.1.1 Acid-base accounting (ABA) tests
Acid-base accounting (ABA) is commonly used to quantify the long-term potential for a
given rock or waste material to generate acid. A large number of ABA testing
procedures have been developed over the past 3 decades (White et al., 1999; Mills, 2007).
The basic idea is to compare the acid-generating potential (AP or AGP) of a given rock or
soil sample with its acid-neutralizing potential (NP or ANP). Values of AP and NP are
usually reported in units of kg CaCO
3
/ton of rock. The neutralization potential ratio
(NPR) is simply NP/AP. A general rule of thumb is that if a sample has an NPR < 1 then
it is likely to generate acidic leachate, whereas if the NPR > 3, then it is unlikely to
generate acid. Samples with NPR between 1 and 3 may or may not generate acid,
depending on the abundance and relative rates of weathering of the various mineral
components, among other factors.
The acid-generating potential (AP) of a sample is usually determined by measuring the
concentration (wt %) of sulfide-S in a given volume of sample. A common assumption is
that each mole of S in the rock or soil sample will generate 2 moles of acid upon
weathering (see reaction 1). However, this assumes that all of the sulfide-S in the rock is
present as pyrite. Although pyrite is by far the most abundant sulfide mineral in the
Earth’s crust, it is by no means the only one. Some other common sulfide minerals and
their acid-base behaviors during weathering are listed in Table 2.
The most widely-used method for NP determination is the Sobek test (Sobek et al.,
1978), which was developed by the US-EPA. In brief, this procedure entails reacting a
known volume of crushed rock or soil sample with a known volume of acid, and then
determining by titration the amount of acid consumed by the rock. By far the most
reactive sources of NP in a rock are carbonate minerals (e.g., calcite, dolomite), although
some silicate minerals such as plagioclase and olivine can also help to neutralize acid (see
Section 2.3.2.6). There are many variants of the original Sobek method being used by
environmental laboratories, and for this reason it is a good idea for a client to get
documentation of laboratory procedures when NP analyses are performed.

41
Table 3: A list of some common sulfide minerals and their likelihood to generate acid on
weathering
.
mineral formula
Behavior on weathering
pyrite FeS
2
Acid-producing
pyrrhotite FeS Acid-producing
chalcopyrite CuFeS Acid-producing
bornite Cu
5
FeS
4
Acid-producing
arsenopyrite FeAsS Acid-producing
sphalerite ZnS Non acid-producing
galena PbS Non acid-producing
chalcocite Cu
2
S Acid-consuming
A big advantage of ABA tests (also known as “static” tests) is that large numbers of
samples can be processed and analyzed relatively quickly and cheaply. It is not
uncommon for a mining operation to generate several hundred individual ABA analyses.
This information is then used to make decisions regarding management of mine waste,
both during and after the mine life. A major disadvantage of the ABA tests is that they
give no information on the rates of reactions that lead to acid production or consumption
in the field. They also give little or no information on chemistry, such as the
concentrations of toxic metals that are likely to be present in a mine waste leachate.
3.1.2 Kinetic tests
Kinetic tests are often employed to get more detailed information on the chemistry of
mine waters that are likely to be generated in different mine waste settings. One common
procedure is the so-called “humidity cell” test (Lapakko, 2003; ASTM, 2007). A
humidity cell is a plastic cylindrical chamber filled with 1 kg of crushed rock. The cell is
fashioned with ports at the top and bottom for addition of water and/or compressed air.
Humidity cell tests are performed on a one-week cycle, where dry (de-humidified) air is
circulated through the sample for 3 days, followed by 3 days of humid air. On the 7
th
day
distilled water is added. After 24 hours the sample is drained and the leachate is
collected for chemical analysis. This procedure is repeated for a long time, typically > 1
year. The data obtained can be used, among other things, to determine the relative and
absolute rates of sulfide depletion and NP depletion from the sample.
In addition to laboratory humidity cell tests, there are a number of possible field tests that
can be set up – usually during the period of active mining operations – to help predict
leachate chemistry. These include field test plots of waste rock piles or tailings dumps,
as well as minewall runoff collection systems (Morin and Hutt, 2001). The big
advantage of field kinetic tests is that they generate data on weathering rates and leachate
chemistry in situ, under conditions of climate and microbial activity that are similar to the
mine site.

42
3.1.3 Pit lake mesocosm experiments
Some simple but useful information to predict future pit lake chemistry can be obtained
by gathering representative bulk samples of weathered bedrock from different parts of the
pit-wall, crushing the samples to a gravel size, and then leaching with distilled water in
the laboratory. This is similar to the leaching procedure used in a humidity cell test.
Although crude, these “mesocosm” experiments (sometimes referred to as “pit lake in a
can” experiments) are a simple, inexpensive way to get an initial idea of future mine
water quality (Davis, 2003). For example, Newbrough and Gammons (2002) performed
mesocosm experiments on weathered bedrock from the Berkeley Pit and showed that the
leachate obtained had a major and trace metal chemistry remarkably similar to that of the
Berkeley pit lake. If there are several different rock types exposed on the walls of an
open pit mine, it may be possible to mix a proportionate mass of each unit into a single
batch vessel and then measure the water quality of the ensuing leachate.
3.2 COMPUTER MODELING
3.2.1. Hydrogeological models
If a pit lake is part of the final plan for a closed mine, it is critical to have a good idea of
how fast it will fill (Naugle and Atkinson, 1993). There are many ways to approach this
problem, with differing levels of complexity. If the lake is slated to be filled rapidly
(e.g., in a few years or less) by diverted surface water or rapidly pumped groundwater,
then estimation of the rate of filling is straightforward, and can probably be done with a
simple spreadsheet. The short-term water quality of the lake can be approximated if the
chemical composition of each end-member water is known, and if the mixing ratio of the
different waters is known. This type of mixing calculation can be done empirically via a
laboratory experiment, or numerically using a geochemical modeling program (see
below). Using relatively simple analytical equations, it is also possible to come up with
initial estimates of the projected rate of groundwater inflow into an open pit (Hanna et al.,
1994; Marinelli and Niccoli, 2000; Shevenell, 2000b).
For more sophisticated modeling, especially for long-range predictions or in cases where
the lake is expected to fill slowly (e.g., > 10 years), a 3-dimensional groundwater flow
model will be needed to predict the rate of groundwater seepage into or out of a pit lake.
One example of a program with this capability is MODFLOW, developed and supported
by the U.S. Geological Survey (USGS, 2000). MODFLOW requires a large amount of
site-specific information including climate, topography, sources of groundwater recharge
and discharge, and information on the hydrogeologic properties of geologic units in the
subsurface, including both the vadose zone and zone of saturation. Two basic types of
model can be created: static or transient. With a static model, one synthesizes and
arranges input data until the predicted distribution of hydraulic head at all points in the
system agrees with the measured hydraulic heads (e.g., from groundwater monitoring
wells). Once calibrated, the static model can be expanded to a transient model that has
the ability to simulate changes in the potentiometric surface of groundwater in response
to changes in climate or land use.

43
3.2.2 Limnological models
Limnological models are used to predict vertical stratification in lakes, and the frequency
and depth to which lake turnover is likely to occur. Probably the most common
limnological model currently in use by pit lake researchers is the program DYRESM,
which stands for Dynamic Reservoir Simulation Model (Imberger and Patterson, 1991).
DYRESM is a one-dimensional model that is most commonly used to predict seasonal
changes in the vertical density profile of a lake. Like MODFLOW, a large amount of
site-specific information is required to create an input file. The exact morphometry (i.e.,
size and shape) of the open pit is first entered into the data base. Detailed seasonal (e.g.,
hourly or daily) meteorological information is needed, including precipitation, air
temperature, relative humidity, wind direction and wind speed, and solar radiation.
Because the climate inside a mine pit can be different from that in the surrounding local
region (especially with regards to wind direction and wind speed), meteorological data
collected within the pit near the lake surface is best. The initial salinity and temperature
depth-profile of the lake must be specified, as well as the volume, temperature, and
salinity of any groundwater or surface water inputs or outputs from the lake. With this
information, DYRESM then calculates the future vertical density profile of the lake in the
desired time-step (e.g., days or weeks). Apart from keeping track of salinity (which is
needed to calculate water density), DYRESM does not deal at all with chemistry. A copy
of DYRESM and a user’s manual can be downloaded from the Center for Water
Research at the University of Western Australia (CWR, 2007).
3.2.3 Geochemical models
Geochemical models are frequently used by pit lake researchers to determine the aqueous
speciation of water in a lake and to predict possible chemical reactions, such as mineral
dissolution/precipitation or adsorption of dissolved solutes onto mineral surfaces. Alpers
and Nordstrom (1999) give a good review on the subject, including an overview of the
different computer codes that are available. Two of the more commonly used modeling
programs include Visual MINTEQ and PHREEQC. MINTEQ was developed by the
U.S. Environmental Protection Agency (EPA), and has a large thermodynamic database
for dissolved and solid inorganic substances, including many toxic metal compounds.
Visual MINTEQ (KTH, 2007) is a user-friendly, windows-based version of the original
MINTEQ program. PHREEQC is available with extensive documentation from the
USGS (USGS, 2007). Of the two programs, PHREEQC has more advanced capabilities,
such as inverse geochemical modeling, 1-dimensional transport, batch reaction (including
evaporation), and stable isotope mass balance. For routine geochemical calculations,
there is little difference between the results obtained by either program. It is important
to realize when using a geochemical model that the results are only as good as: 1) the
quality of the input data (e.g., laboratory analyses); and 2) the accuracy and completeness
of the thermodynamic data in the program database. Both Visual MINTEQ and
PHREEQC have periodic updates to their databases which can be downloaded from their
respective websites.

44
3.2.4 Biological models
There have been relatively few attempts to model the biology of mining pit lakes. The
University of Western Australia (UWA) has developed a program called CAEDYM, or
Computational Aquatic Ecosystem Dynamics Model, which can be used to quantify
biological influences on the water quality of lakes, including pit lakes. Some of the
processes that are modeled include primary and secondary production rates, nutrient and
metal cycling, oxygen production and consumption, and transport of particulate organic
carbon. It is also possible to couple CAEDYM with DYRESM, as described at the UWA
website (CWR, 2007).
The biotic ligand model (BLM) has recently been developed to better quantify the
toxicity of various heavy metals to aquatic life (see Paquin et al. (2002) and Niyogi and
Wood (2004) for good overviews of the BLM). Specifically, the BLM calculates the
level of metal accumulation at the site of action of acute toxicity – referred to as the
“biotic ligand” – in an aquatic organism (Paquin et al., 2002; Niyogi and Wood, 2004).
For fish, the biotic ligand for the majority of metals of concern is located within the gill
(Paquin et al., 2000). When the concentration of a metal toxin at the biotic ligand site
exceeds a particular threshold, then mortality may ensue (Di Toro et al., 2001). An
important aspect of the BLM is that it includes thermodynamic data to compute the
aqueous speciation of each metal of interest for any site water chemistry (DiToro et al.,
2001; Paquin et al., 2002; Niyogi and Wood 2004). Parameters such as pH, hardness,
and DOC concentration are important variables that influence the aqueous speciation, and
therefore the bioavailability, of each dissolved metal (see section 2.5.5.1). To date, the
BLM has most frequently been used with fish and crustaceans to predict toxicity of some
of the more common heavy metals, such as Cu and Zn. In the future, additions to the
program will allow the BLM to speciate more metals of interest, and to be applied to
more organisms (e.g., green algae and bacteria) (Paquin et al., 2002). The BLM may
become an important tool to predict the biological impacts of water quality in pit lakes,
especially if the potential for establishment of a fishery exists.
4. ENHANCING BENEFICIAL END USES FOR PIT LAKES
The concept of incorporating beneficial end uses for pit lakes in drafting mine closure
plans is relatively new. There are at least some examples of mining pit lakes that were
flooded many years ago and now have high recreational and ecological value. These
include – among others – a number of large lakes in northern Minnesota and southern
Ontario left behind from open pit iron ore mining. It is much more difficult to see a
benefit to acidic, metal-rich lakes such as the Berkeley pit lake in Butte, Montana, and yet
even this water body has some beneficial uses, as will be discussed in the following
sections.

45
4.1 SUMMARY OF BENEFICIAL END USES
Possible beneficial end uses for mining pit lakes were recently reviewed by Doupé and
Lymbery (2005) and McCullough and Lund (2006). Table 3 summarizes concepts
presented in these two papers, with some additional ideas. Each of these end use
categories is discussed in more detail in the following sections.
Table 4. Summary of beneficial end uses for pit lakes, with examples.
Beneficial end use
Examples
1 Permanent storage of
reactive mine waste
many
2 Water supply for industry
or agriculture
Illinois coal mines (GE78); Enterprise Pit, Northern
Territories, Australia (SF94)
3 Aquaculture Highland Valley, BC (O04); Brazilian Amazon
(O04); Minnesota (A96, A98, Y97)
4 Functional habitat for
aquatic life
Highland Valley, BC (O04); East Pit Lake, AB
(S95); Minnesota (A96a, Y97); Gunnar, SK (T82),
Steep Rock, ON (S04); Lac des Roches, AB (S02)
5 Recreation and tourism East Pit Lake, AB (S95); Portsmouth, MN; Steep
Rock, ON (S04); Berkeley Pit, MT
6 Metal recovery Berkeley Pit, MT (GD06)
7 Heat pump applications none
8 Scientific study many
References: A96 = Axler et al. (1996); A98 = Axler et al. (1998); GD06 = Gammons and
Duaime (2006); GE78 = Gibb and Evans (1978); O04 = Otchere et al. (2004); S02 = Schwartz
(2002); S04 = Sowa (2004); SF94 = Sinclair and Fawcett (1994); S95 = Sumer et al. (1995); T82
= Tones (1982); Y97 = Yokom et al. (1997)
4.1.1 Storage of mine waste
Probably the most common use of abandoned open pits is the permanent disposal of mine
waste (e.g., waste rock or mill tailings). The concept of putting waste rock back into the
pit it came from (i.e., backfilling) is compelling from the point of view of minimization
of long-term disturbance of the land. It can also decrease the volume and severity of acid
mine drainage at a closed mine site by burying reactive waste. However, it is stressed
that backfilling of open pits is not always the best strategy. Each mine closure plan must
be evaluated on a case-by-case basis.
Figure 18 summarizes some possible backfill options for an open pit that is dug into a
hillside. Backfill can be complete (Fig. 18a) or partial (Fig. 18b), and the latter can be
designed with or without a pit lake. Ideally, any reactive waste (i.e., material that is
likely to generate acid or metal-rich leachate) will be isolated from surface water by a
cover of non-acid-generating waste rock or clean fill. Oxidation of sulfide minerals will
normally be negligible if the reactive waste is permanently submerged or saturated with
groundwater. However, if any sulfide-rich rock is left exposed on mine walls above the
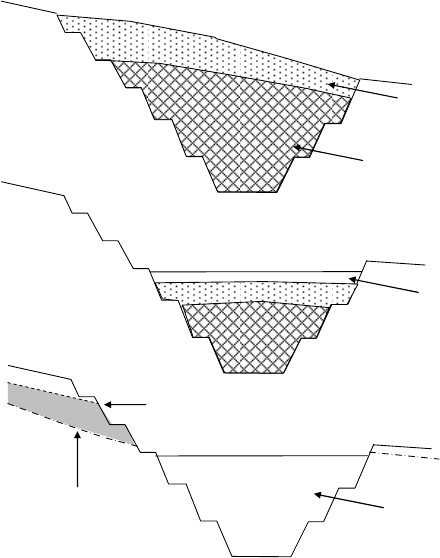
46
lake surface or water table, then oxidation and weathering of this material could seriously
compromise the long-term water quality of the lake and surrounding groundwater. In
figure 18, the most likely areas of concern would be the highwalls on the left-hand side of
the diagrams in “b” and “c”. In both cases, the post-mining, steady-state water table
would be permanently drawn down on the left-hand highwall, due to the modification in
the topography. This would result in long-term exposure of reactive rock to weathering
and pyrite oxidation. To minimize problems of this sort, clean fill or non-reactive waste
rock could be bulldozed over the exposed walls of the pit to cover the offending rock
types. However, this will not completely stop pyrite oxidation in the “wedge” of de-
watered rock between the pre-mining and post-mining water tables (see Fig. 18c).
Figure 18. Diagram showing different possibilities for backfilling a mining pit on a
hillside: a) complete backfill; b) partial backfill with a shallow lake; c) no backfill with a
deep lake. The shaded region in “c” shows a wedge of permanently dewatered bedrock
where acid mine drainage is likely to occur.
A common procedure when back-filling is to blend lime (CaO) or limestone (CaCO
3
)
into reactive waste rock as it is placed back into the pit. In this case, the amount of lime
or limestone added to each truck load of waste is determined by acid-base accounting.
Some back-fill designs include a layer of crushed limestone near the bottom to neutralize
any acid leachate that may drain from the waste pile after closure. Another option is to
lay down a highly permeable gravel layer at the bottom of the pit to divert any influent
groundwater to a subsurface pipe or pumping well before it can reach the pyrite-bearing
waste rock, thereby discouraging contact between groundwater and reactive waste. This
type of design would only be appropriate for open pits in relatively dry climates where no
lake is expected after closure.
Reactive waste
Clean fill,
topsoil
lake
lake
b.
a.
c.
post-mining water table
pre-mining water table

47
4.1.2 Water supply
In northern Canada, there are relatively few opportunities for a flooded open pit mine to
have beneficial use as a water supply, given the abundance of natural lakes and rivers in
the Canadian Shield. One possible use of a pit lake would be as a supply of water for the
construction of ice roads that are essential for heavy equipment to navigate the tundra
(Cott et al., 2008b). Use of water from natural lakes could result in drawdown of the
water surface, with possible negative impacts to native fish and other fauna. In contrast,
pit lakes in arid or semi-arid parts of the world, such as Australia or the Great Basin of
the USA, could represent a major local water resource with a number of possible end
uses, depending on water quality (Sinclair and Fawcett, 1994; Doupé and Lymbery,
2005; McCullough and Lund, 2006). Possible uses include irrigation of crops or lawns
(including golf courses, municipal parks, playing fields, etc..), irrigation of livestock,
industrial uses, and public drinking water supplies. The latter would predictably have the
most stringent water quality requirements. One example of a pit lake that is being used
for drinking water is the city of Astoria, Illinois (USA) which since 1976 has been
treating water from an abandoned open pit coal mine for drinking water (Gibb and Evans,
1978). Although water quality standards for irrigation of plants or livestock are typically
less strict than those for human consumption, irrigation is sensitive to high salinity
(especially high Na concentrations) and the presence of toxic substances, such as heavy
metals or arsenic. Water quality requirements for industrial use will vary, depending on
the nature of the commercial process involved. Given the fact that most mines in Canada
are in remote locations far from industrial centers, it is unlikely that this would be a major
beneficial use.
4.1.3 Ecological Habitat
Ideally, a “restored” pit lake will be a fully-functioning aquatic ecosystem, including
fauna such as macroinvertebrates, fishes, and waterfowl. For example, Brenner et al.
(1987) describe a large number of coal mine pit lakes in Pennsylvania that contain a
diverse ecological community, including fishes, which reflects favorably on the quality of
habitat provided by these waterbodies. Since pit lakes may in favorable circumstances be
capable of supporting a diverse ecological community, the ecological benefits of these
lakes must be considered during the reclamation process, especially in terms of habitat
creation or restoration and the maintenance of such habitat. As discussed in Section 2.5,
biodiversity of a newly-formed pit lake is likely to be low, but is expected to increase
with time, and can furthermore be enhanced by re-landscaping the pit walls or treating
the contained water, either chemically or biologically (see Section 4.2). As well, steep,
rocky high walls above the lake surface can be potential habitat for birds, bats, rodents,
and more charismatic animals such as mountain goats or wild sheep. For example, the
authors of this report have observed bighorn sheep (Fig. 19) and cliff swallows inhabiting
closed open pit mines in Montana.

48
Figure 19. Big horn sheep (Ovis Canadensis) inhabiting a reclaimed open pit gold mine
in Montana (photo by Chris Gammons, 2007).
There are also possible negative consequences of pit lakes from the standpoint of habitat.
For example, in arid, low-latitude regions (western Australia, SW USA, Africa), pit lakes
may be breeding grounds for disease-carrying insects, such as mosquitoes (Norris, 2004;
Doupé and Lymbery, 2005). Blooms of toxic blue-green algae or other poisonous
phototrophs are also possible, especially for lakes that have been artificially nutrified.
In a recent review paper, Nixdorf et al. (2005) propose a new ecological classification
scheme for pit lakes based on acidity and phytoplankton species and abundance. Nixdorf
et al. (2005) suggest that pit lakes with low to moderate acidity have a good chance of
being restored to a beneficial ecological end use through in situ treatment and long-term
management of nutrient budgets. Interestingly, these same authors suggest that at least
some pit lakes that are strongly acidic should be preserved as habitat for extremophilic
microorganisms. However, if preservation of acidic lakes is the intended end use, then
steps must obviously be taken to insure the protection of downstream water quality, and
also to avoid accidental death of animals that may come into contact with the acid waters.
In November of 1995, Butte, Montana became the focus of media attention when 349
migrating snow geese were found dead on the surface of the Berkeley pit lake (Adams,
1995). To avoid a recurrence of such an event, the lake is now equipped with a system of
loud acoustic emitters to “haze” ducks and geese who may otherwise land on the water.
There is also a permanent rifle shed mounted near the pit rim so that mine operators can
fire warning shots to discourage waterfowl from landing on the lake.

49
4.1.4 Aquaculture
As summarized in the optimistic article by Otchere et al. (2004), the commercial or
recreational use of pit lakes for aquaculture (fish farms) could be an innovative way to
enhance the ecological, economic, and cultural sustainability of open pit mining. Pit
lakes may in fact be more suitable for aquaculture in comparison to natural lakes since
the former usually have no surface connection to any other water body, thereby
eliminating the risk of introduction of non-native fish or other negative consequences of
fish farms (e.g., disease, antibiotics, fertilizers) into the surrounding aquatic ecosystem
(Otchere et al., 2004; Naylor et al., 2005). By virtue of their great depth and (often)
meromictic characteristics, pit lakes have the potential to permanently sequester nutrients
and decaying organic carbon in benthic sediment, thereby removing sources of biological
oxygen demand that otherwise can plague aquaculture practices. On the other hand,
there are several examples where extensive aquaculture in a mine lake had negative
impacts on water quality due to eutrophication from nutrient overload (Yokom et al.,
1997; Axler et al., 1996, 1998). The possibility of nutrient imbalance or spread of a
pathogen-borne disease from aquacultural activities is made more likely in the case of a
pit lake that has no outlet, due to the lack of any throughput of fresh water.
4.1.5 Recreation and tourism
If water quality is good and there are no site-specific safety concerns, there is no a priori
reason why a pit lake could not be used for public recreation, such as swimming, boating,
scuba-diving, fishing, or hunting for waterfowl. The numerous lakes left behind from
iron ore mining in northern Minnesota, Wisconsin, and Ontario are good examples (see
Section 5.2, below). Many people are fascinated by geographic oddities, and therefore a
large pit lake could actually benefit the local community as a tourist attraction, even if it
is widely considered to be an environmental disaster. Strange as it sounds, the famously
toxic Berkeley pit lake is the largest tourist attraction in Butte, Montana, an historic
mining center that is struggling to make the transition from resource extraction to a more
diversified economy including tourist dollars as a major source of income. Another
example is the locally-famous “Big Hole”, which is the site of the original Kimberley
diamond mine in South Africa, which ceased operations in 1914 and now contains a pit
lake. Although no scientific papers on this pit lake could be found, some touristic
information is available at: http://www.thebighole.co.za/HOME.HTML
. A photo of this
historically-important mine and pit lake is given in Figure 20.

50
Figure 20. The “Big Hole” pit lake at Kimberley, South Africa. (Source: Wikipedia.org;
photo by Rudolph Botha, March, 2005).
There are several examples in the literature highlighting the various recreational
opportunities provided by pit lakes, with sport fishing opportunities being one of the most
prevalent. Sport fishing is often a major contributor to the tourism economy of an area,
therefore pit lakes that provide fishing opportunities can also contribute to the local
economy and countless examples exist in the literature. In Pennsylvania, Brenner et al.
(1987) surveyed 60 surface mine lakes in detail and found that many of the lakes were
quite abundant in a variety of local sport fish species, although for the most part these
water bodies were underutilized in regards to recreational fishing because of land access
issues. In Alberta, the development of sport fisheries in lakes created by coal mining
operations in the eastern slopes has become a common practice (Luscar Ltd., 1993).
Several of these reclaimed coal mines that subsequently filled with water to create lakes
are presently inhabited by native and nonnative fish species, all of which provide
excellent sport fishing opportunities (Schwartz, 2002). Establishing sport fisheries in pit
lakes is expanded upon in more detail in section 4.5.2.
Given favourable water quality conditions, wildlife may also be frequent visitors to, or
common inhabitants of, pit lakes, and in such cases these waterbodies could provide
exceptional wildlife viewing opportunities. As mentioned in section 2.4., waterfowl may
often be common users of pit lakes (Phylips, 1992), and such a scenario would provide
bird enthusiasts with a chance to view this wildlife. Additionally, opportunities may also
exist for the viewing of other wildlife that are often associated with aquatic environments
such as muskrat (Ondatra zibethicus), beaver (Castor canadensis) and potentially even
large ungulates such as moose (Alces alces), although no literature was found to

51
document this. Additionally, depending on the species and local regulations, hunting
opportunities may exist for the above mentioned wildlife.
4.1.6 Metal recovery
Using an acidic, metal-rich pit lake for economic metal recovery can be a silver lining for
an otherwise disastrous environmental situation. Besides being the city of Butte’s main
tourist attraction, the Berkeley pit lake is also a significant money-maker for the local
mining company. At the time of this writing, water in the monimolimnion of the pit lake
is being pumped at a rate of over 10
7
L/day to a copper recovery plant, where the
majority of the dissolved Cu in the water is removed by a process referred to as
cementation (Gammons and Duaime, 2006). The overall reaction is very simple:
Cu
2+
+ Fe(s) → Fe
2+
+ Cu(s) (27)
Water containing 150 to 200 mg/L dissolved Cu is contacted with scrap iron for several
hours, during which time > 80% of the Cu is extracted in elemental form. The cemented
scrap is washed and mechanically separated to obtain a copper metal concentrate, which
is then shipped for refinement. Meanwhile, the Cu-depleted water is returned to the pit.
This process has been producing over 10
6
kg of Cu annually since 2003, but will
eventually slow down or perhaps cease as the dissolved Cu in the pit lake is “mined out”.
An important question for the mining company is whether or not dissolved Cu
concentrations in the pit lake will ever return to economically-exploitable levels with
time. If so, then the copper recovery circuit would be an interesting variant of the heap-
leach method of Cu-recovery that is commonly used for low-grade, bulk tonnage
porphyry copper mines. There have been intermittent attempts at recovering other
metals from the Berkeley pit lake. For example, concentrations of dissolved Zn in the
lake (~ 600 mg/L) are even higher than Cu. However, an economically-viable
technology for Zn recovery is yet to be developed.
4.1.7 Heat pump applications
By virtue of their immense volumes and proximity to mining-related infrastructure (mills,
offices, employee residences, etc...), there may be potential to use pit lakes as heat
exchangers for space heating and/or air conditioning. Heat pump technology has
advanced in the past few decades, and it is now possible to heat or cool large buildings
with groundwater or surface water that is near ambient temperature. There are several
examples of projects where the potential for heat to be recovered from flooded
underground mine workings is being evaluated (Arkay, 1992; NETL, 2006; Watzlaf and
Ackman, 2006; Minewater Project, 2007), including in northern Canada (Ghomshei,
2007). Realistically, heat recovery from pit lakes may be more difficult owing to the
relatively cold temperatures of most stratified lakes below the thermocline. However,
with improved technology this may be something to look into, especially in frigid
climates.

52
4.1.8 Scientific study
Mining pit lakes represent a unique opportunity for scientific study of limnological
systems that – in many cases – have highly unusual morphometry, biology, chemistry,
and hydrology. Detailed investigations of pit lakes have the potential to yield new
advances in microbial ecology, biometallurgy, and biochemical engineering. For
example, the Stierle research team at Montana Tech have been isolating and testing
unique secondary metabolites from the Berkeley pit lake for possible cures for human
diseases, including cancer and AIDS (Stierle and Stierle, 2005). Investigation of the
microbial ecology of the Berkeley Pit and other acidic water bodies may even have
applications to the emerging field of astrobiology. This is especially true given recent
evidence collected from NASA missions for the past existence of acidic lakes and
groundwaters on the surface of Mars (Squyres and Knoll, 2005).
4.2 STRATEGIES FOR ENHANCING END USES
4.2.1 Landscaping and backfilling
It is becoming more common during mine closure to change the profile of abandoned
open pits to optimize end use strategies. Many open pit mines have extremely steep
profiles: 1:1 slopes are not uncommon. Steep slopes of blasted bedrock and overburden
are highly unstable, leading to mass wasting (creep, landslides) and making it virtually
impossible to re-vegetate the mine walls. In addition, a uniformly steep-sided, deep lake
lacking any littoral habitat (shallow water along shore lines) is undesirable from the
standpoint of biodiversity and primary production by rooted macrophytes and benthic
algae (BHP, 1999). A number of possible actions could help rectify this problem,
including bulldozing of upper slopes into the pit, partial backfilling with mine waste (see
section 4.1.1), and excavation of rock or soil along pit walls to create artificial habitat
such as littoral bays, reefs, or shallow spawning beds (BHP, 1999).
It should be obvious that any large-scale re-landscaping of an open pit is best performed
as soon as possible after mine closure, ideally by the mine operators themselves.
Bulldozing large volumes of unconsolidated rock and soil into a lake that is already at
full stage may be impossible without a safe way to discharge the displaced water. In
addition, covering reactive, pyrite-bearing rock with overburden immediately after the
mine is closed will lessen AMD impacts. Given the fact that mining companies are
particularly adept at moving large amounts of rock quickly, failure to take these steps at
the end of the mine life may be an opportunity missed.
4.2.2 Rapid flooding
Left to their own devices, pit lakes can take a very long time (decades to 100s of years) to
flood completely (Section 2.2). This is undesirable, as it gives more time for natural
weathering processes to deplete the rocks exposed on the walls of the pit of their

53
neutralization potential, increasing the likelihood of severe acid mine drainage. To
minimize the risk of AMD, it is generally recommended to flood open pits as rapidly as
possible. Depending on the mine location, the source water for flooding could be ocean
water (Fisher and Lawrence, 2006), a nearby reservoir or lake (Tones 1982), a river
(Lessmann et al., 2003), or groundwater that is pumped into the lake at a rapid rate
(Dowling et al., 2004). The source water should have good quality (e.g., low TDS and
near-neutral pH), and if it contains elevated bicarbonate alkalinity then this is a bonus.
On the other hand, if the end lake is designed to be meromictic, then it may be beneficial
to flood the lower portion of the pit with high-TDS water, such as sea water or saline
groundwater, and then flood the upper portion of the pit with lower density, low-TDS
water. Such a design was used for the Island Copper pit lake on Vancouver Island, BC
(Fisher and Lawrence, 2006).
If rapid flooding of a pit lake is planned, steps should be taken to ensure that a potentially
harmful drawdown of the source water body is avoided (McAfee, 1980; Gaboury and
Patalas, 1984; Mills et al., 2002). A rapid drop in the elevation of a natural lake could
lead to erosion and re-suspension of unconsolidated lacustrine sediment, with a resultant
sharp increase in turbidity of the water column. Lake drawdown could also expose high-
quality littoral habitat to open air and – in the case of northern Canada – freezing
temperatures. Rapid removal of water from shallow parts of frozen, natural lakes could
also deprive over-wintering fishes of O
2
-rich water in the epiliminion (Cott et al., 2008b).
In some special cases, it may be possible to flood an abandoned open pit mine with mill
tailings waste from a nearby active mine. Because tailings are usually > 70% water, a
mine void flooded in this manner would develop a sizeable lake with a thick pile of
unconsolidated metalliferous sediment at the bottom. The authors are not aware of a pit
lake that has been formed in this manner, although examples probably exist. A much
more common practice is to discharge mine tailings into engineered repositories or to
pre-existing natural lakes or ocean basins. There are several possible advantages of
filling a mine pit with tailings, including: 1) flooding is likely to be rapid given the large
volume of mill tailings generated each day at most large mines; 2) solid waste is
permanently disposed of as partial backfill in the bottom of the lake, obviating the need
for a separate tailings impoundment; and 3) due to pH adjustment in the mill, most
tailings waters are strongly alkaline (pH > 10): the additional alkalinity could offset acid
from AMD and help to buffer the final pH of the lake to neutral or weakly alkaline
values. Possible disadvantages include uncertainty as to the final pit lake water quality,
as well as the need for an alternate supply of make-up water for the mill during active
mining operations and a multiple-pit mine plan.
4.2.3 Chemical Treatment
Chemical treatment of pit lakes is most often used to make pH adjustments. This can be
done gradually, while the lake is flooding (Dowling et al., 2004), or in a single- or
multiple-phase treatment operation after the lake is full (Peine and Peiffer, 1998; Lewis et
al., 2003). Both of these scenarios are in situ treatment schemes, meaning that lime or
54
some other alkaline reagent is added directly to the lake. Addition of lime to an already
flooded lake is usually accomplished from a boat or barge, and is complicated by the
possibility of incomplete vertical and/or horizontal mixing of lime into the water column.
In some cases, chemical treatment of pit lake water is accomplished ex situ by pumping
water from the lake, passing through a lime treatment plant, and then discharging the
clean water to a receiving water body. Obviously, this type of treatment process will do
nothing to improve the water quality of the pit lake, unless the treated water is returned to
the mine pit to form a closed loop.
The most common reagent used to raise the pH of acidic mine water is lime. Lime is
made from limestone by heating the rock to the point that CO
2
is driven off, leaving an
anhydrous CaO compound behind. Because anhydrous CaO is slow to dissolve in water,
many treatment plants first hydrate their lime to Ca(OH)
2
(sometimes referred to as
“slaked” lime). Depending on cost and availability, other alkaline reagents can be used
for pH adjustment, such as NaOH or MgO. Phosphate-rich rock has also been used to
increase the alkalinity and nutrient content of acidic lakes (Davison et al., 1995).
However, lime is by far the most common reagent used for large-scale treatment
operations.
When lime or slaked lime is added to mine water, the pH is raised as shown in the
following reactions:
CaO(s) + 2H
+
→ Ca
2+
+ H
2
O (28)
Ca(OH)
2
(s) + 2H
+
→ Ca
2+
+ 2H
2
O (29)
Depending on the starting chemistry of the water, a number of side-reactions can occur as
the pH increases. These include precipitation of gypsum (CaSO
4
·2H
2
O), calcite, and/or
hydrous oxides of Fe and Al, along with co-precipitation or adsorption of trace metals
and metalloids (e.g., arsenic). Together, the solid precipitate that forms from lime
treatment is usually referred to as “sludge”. In aerobic environments (DO present), lime
treatment sludge is usually brown or red, reflecting the presence of HFO. This type of
sludge is notoriously slow to settle out of the water column, and may impart a strong
turbidity to the water (Fig. 21a). In anaerobic environments (DO absent), sludge can take
on a greenish color (Fig. 21b) due to the presence of a metastable, Fe
II
-bearing compound
known as “green rust”. Green rust has a range of chemical composition, but when
formed from initially acidic, sulfate-rich water it can be approximated by
Fe
II
4
(Al,Fe
III
)
2
(OH)
12
SO
4
· 3H
2
O (Randall et al., 2001). Like HFO, green rust has a high
affinity for trace metals and metalloids, and therefore precipitation of this compound can
strip other toxins from the water column. Unlike HFO, green rust is quick to settle by
gravity through the water column, and therefore has superior properties from the
standpoint of turbidity. Unfortunately, it is unstable in the presence of oxygen, and will
rapidly break down if exposed to air or water containing elevated dissolved oxygen. The
conversion of green rust to Fe(III) compounds releases acid, and may drop the pH of
surrounding waters. Thus, fore-thought needs to be given as to how any “green rust”
sludge is permanently disposed of in a pit lake setting.

55
Figure 21. Photographs of lime treatment sludge: a) plume of hydrous ferric oxide in the
surface of the Berkeley pit lake formed by disposal of alkaline lime treatment sludge
(photo by Chris Gammons, June, 2006); b) “green rust” formed by rapid pH increase of
Fe
II
-rich mine water (Chris Gammons).
The optimal end pH during lime treatment of mine water depends on the initial water
chemistry and which solutes are problematic. Most trace metals (e.g., Cd, Cu, Mn, Ni,
Pb, Zn) are removed efficiently at pH’s in the range of 9 to 10 (Fig. 22), whereas the
common rock-forming metals Fe and Al are effectively removed at lower
pH’s (e.g., 6 to 8). The fact that the solubility minimum of Al occurs at a lower pH than
those of the trace metals creates a dilemma for lime treatment engineers: namely,
adjustment of pH to values > 9 is needed for complete Zn removal, whereas this can
result in elevated dissolved Al concentrations. For this reason, many treatment plants
employ a “two-stage” lime addition where the pH is first adjusted to ~ 7 to precipitate an
Fe-Al sludge which is thickened and removed. The water is then passed into a second
tank where pH is raised to 9 or higher to remove trace metals. Unfortunately, this type of
two-stage lime addition is not really feasible for in situ lake treatment. This is because
the Fe-Al sludge formed at neutral pH may back-react following later adjustment of pH
to values > 9, releasing Al to the water column. Benchtop experiments and/or
geochemical modeling are recommended to sort out all these complications beforehand.
Finally, it is important to consider what pH is optimal for micro- and/or macro-fauna,
assuming that this is an important component of the end use of the lake. For example,
many fish species including salmonids are sensitive to strongly alkaline pH (> 9). High
pH interferes with the fish’s ability to excrete ammonia and maintain a proper electrolyte
balance (Wilkie et al., 1996). On the flip side, trout and other fishes are also highly
sensitive to heavy metals, such as Cu, Ni, Pb, and Zn, at very low concentrations (section
a.
b.

56
2.5.5.1). To effectively remove these trace metals from a pit lake while maintaining pH
values below 9 may require alternative strategies, such as biological treatment.
Figure 22. The dependence of the solubility of selected metal-hydroxide minerals on pH.
The “optimal treatment” line is generalized. Water quality standards vary for each metal
and from region to region. Note the high solubility of Fe
2+
relative to Fe
3+
. In mine
waters, Fe
2+
concentrations may be controlled by another less soluble mineral, such as
“green rust” or siderite (FeCO
3
). Diagram modified from Nordstrom and Alpers (1999).
4.2.4 Biological Treatment
Biological treatment of mine water is often referred to as “passive” treatment in reference
to the fact that micro-organisms can often clean up polluted water on their own, with little
or no external stimulation. However, it is also possible to artificially add organic
compounds, such as straw, food waste, or livestock waste, to speed up the rate of
biological activity (Klapper and Geller, 2001; McCullough, 2007). The microbiology of
pit lakes is inherently a very complex subject. For the purposes of this document we will
discuss biological treatment depending on whether conditions are aerobic or anaerobic.
Both bacteria and algae play an important role in aerobic biological treatment, whereas
only bacteria are active in anaerobic conditions.
4.2.4.1 Aerobic biological treatment.
The presence of O
2
in shallow pit lake waters will trigger growth of aerobic bacteria that
use this compound to oxidize Fe
2+
, Mn
2+
, and/or reduced sulfur or nitrogen compounds.
Oxidation of Fe
2+
has already been discussed (section 2.4.2.5). This reaction produces
fluffy precipitates of HFO with high surface area and a strong affinity to adsorb trace
metals. On the negative side, precipitation of HFO releases protons and can lower the pH
of the pit lake if there is insufficient bicarbonate ion dissolved in the water to buffer pH
l
l
l
l
l
l
l
l
l
ll
l
l
l
l
ll
l
l
ll
l
l
l
l
l
l
l
l
l
ll
l
l
Fe(OH)
3
Al(OH)
3
Cu(OH)
2
Fe(OH)
2
Zn(OH)
2
Fe
III
Fe
II
Al
Cu
Zn
pH
2
468
10 12
Metal solubility, molal
10
15
10
10
10
5
1
10
-5
10
-10
optimal treatment

57
to circum-neutral values. Bacterial oxidation of Mn
2+
is much slower than oxidation of
Fe
2+
, but is nonetheless thermodynamically favorable at neutral to alkaline pH (Walton-
Day, 2003). Like HFO, hydrous manganese oxide (HMO) has a very strong tendency to
adsorb trace metals, especially divalent cations such as Zn
2+
and Cd
2+
. Most Fe- or Mn-
oxidizing bacteria are autotrophs, meaning that they use inorganic CO
2
as their source of
C. Growth of autotrophic bacteria therefore constitutes a sub-type of primary production
of organic carbon which, in addition to photosynthesis, can stimulate biological activity
in a lake.
There are several ways in which growth of algae may improve the water quality of a pit
lake. As discussed earlier (Section 2.4.2.2), photosynthesis consumes CO
2
and therefore
will tend to raise the pH of near-surface water, leading to a decrease in the solubility of
trace metals (Fig. 22). In addition, many microorganisms are effective at sorbing metals
directly from solution (Ledin and Pederson, 1996). Although the exact mechanisms by
which metals are taken up by microbes are often poorly understood, it appears that all
algae and bacteria have an ability to adsorb trace metals to binding sites on the exterior of
their cell walls, a process that is metabolism-independent. In other words, metal sorption
occurs whether or not the cells are living. In contrast, “active” uptake implies that the
metal is absorbed into the interior of the cell, where it presumably plays a metabolic role,
e.g., in the construction of enzymes. Either way, algae may effectively “polish” the
water quality of a lake that has chronically elevated concentrations of one or more trace
metals but otherwise good water quality (MERG, 2003; Dessouki et al., 2005). Although
there are many species of algae that can tolerate low pH, algae are less likely to play a
role in cleaning up lakes that are strongly acidic and metal-rich because the
concentrations of protons and dissolved metals are simply too large. It would take an
algal bloom of unprecedented size to significantly impact the chemistry of the Berkeley
pit lake, for example.
Besides raising pH and sorbing metals, algae play an important role in limnic
environments by providing “food” for bacteria and higher life forms. Algae are very
inefficient primary producers, and constantly “leak” photosynthates (e.g., sugars and
other organic molecules) to the water column (Vymazal, 1995). This provides a source
of organic carbon for heterotrophic bacteria. In addition, algae may be grazed by higher
life forms, and as such constitute (along with autotrophic bacteria) the base of the food
pyramid in the lake. Finally, when algae die they sink to the bottom of the lake and
become a source of organic carbon for microbes that promote anaerobic treatment (see
next section).
4.2.4.2 Anaerobic biological treatment
Anaerobic bacteria are likely to dominate the biology of deeper portions of the water
column and underlying sediment in stratified pit lakes. Processes occurring during
anaerobic breakdown of organic matter (see Section 2.4.2.8) include bacterial reduction
of nitrate, iron, and sulfate, as well as fermentation and sometimes methanogenesis.
Amongst this list, the most important reaction from the standpoint of water quality
improvement is bacterial sulfate reduction (BSR). BSR raises the pH of the water, adds
alkalinity, decreases the concentration of sulfate, and promotes precipitation of heavy
58
metals as insoluble sulfide minerals. BSR is the keystone to the majority of engineered
anaerobic passive treatment systems, whether they be in situ or ex situ. On the negative
side, BSR may result in toxic concentrations of free H
2
S, especially if the initial
concentrations of dissolved sulfate are much greater than the combined concentrations of
metals (such as Cu, Fe, Zn) available to form insoluble sulfide precipitates. As well,
BSR has limited ability to attenuate arsenic and a few heavy metals (such as Mn) that
have relatively high solubility in the presence of H
2
S.
With regards to possible end uses for mining pit lakes, the question may well be asked
whether BSR is a good thing or a bad thing. Certainly if BSR is occurring, then there is
zero DO present, which of course means that no animals could survive under these
conditions. Also, if BSR occurs in the monimolimnion or hypolimnion of a stratified
lake, then there is the risk of exposure of organisms to poisonous H
2
S accompanying an
episodic top-to-bottom turnover event (Boehrer and Schultze, 2006). Nevertheless, BSR
is the only biological process that can effectively clean up mine water that is initially
strongly acidic and metal-rich. Most sulfate-reducing bacteria are intolerant of low pH (<
3 or 4) or high concentrations of heavy metals (e.g., > 10 or 20 mg/L of Cu or Zn). In
acidic lakes, sulfate-reducing bacteria most likely gain a foothold in isolated
microenvironments of higher pH, such as may occur around clots of decomposing
organic matter within benthic sediment. The H
2
S produced by the bacteria can then
diffuse upwards into the overlying water column where it precipitates toxic metals as
sulfides, thus creating a more benign environment where the bacteria can spread.
Assuming a replenishable supply of organic carbon is present, this incremental process
could eventually result in a monimolimnion and underlying sediment pore water that is
uniformly sulfidic, with elevated pH and very low heavy metal concentrations. As long
as the lake is permanently stratified, the benefits of BSR probably outweigh the dangers,
especially for pit lakes that otherwise would have very poor water quality.
There have been several published case studies where organic substances were added to a
pit lake (or a lake mesocosm) to stimulate BSR (Klapper et al., 1998; Castro et al., 1999;
Koschorrek et al., 2002; Frömmichen et al., 2003; Fauville et al., 2004). For example, at
the Anchor Hill pit lake of the Gilt Edge mine in South Dakota, US, the pH of the entire
lake was first adjusted by in situ addition of lime (Lewis et al., 2003; Park et al., 2006a,
b). This was followed by injection of a mixture of molasses and methanol to accelerate
BSR and other anaerobic processes. This case study is described in more detail in
Section 5.4. One thing to realize about Anchor Hill is that this lake is relatively small
compared to many mining pit lakes (see Fig. 2). Similar whole-scale manipulations
would be much more difficult and expensive for a larger system, such as the Berkeley pit
lake of Butte, Montana.
Other anaerobic microbial processes that can significantly alter water chemistry include
reduction of iron, nitrate, selenium, and uranium. Bacterial reduction of Fe
3+
to Fe
2+
consumes protons and can significantly raise the pH of deep lake water or sediment pore
water (see Section 2.4.2.8). Like BSR, bacterial Fe reduction can be stimulated by
addition of organic amendments and/or lime (Wendt-Potthoff et al., 2002). Possible
negative consequences of iron reduction include an increase in the total dissolved Fe

59
concentration (since Fe
2+
is much more soluble than Fe
3+
, see Fig. 20), and release of
trace metals or metalloids that were adsorbed onto surfaces of the solid ferric compounds.
Bacterial reduction of nitrate could be beneficial if nitrate concentrations are a concern.
However, if Eh conditions are strongly reducing (e.g., H
2
S stable), this process is likely
to result in elevated concentrations of dissolved ammonia (NH
4
+
or NH
3
) which may be
more problematic than the nitrate itself (Park et al., 2006a, b). On the positive side,
anaerobic environments typically result in near quantitative removal of soluble Se
VI
(SeO
4
2-
) and U
VI
(UO
2
2+
), through precipitation of elemental Se and uraninite. These
reactions can be written as follows:
2SeO
4
2-
+ 3CH
2
O + 4H
+
= 2Se(s) + 3CO
2
+ 5H
2
O (30)
UO
2
2+
+ ½CH
2
O + ½H
2
O = 2UO
2
(s) + 2H
+
+ ½CO
2
(31)
Ample evidence exists for the microbial catalysis of reactions 30 and 31 in anaerobic
environments (e.g., Oremland et al., 1989; Lovley and Phillips, 1992).
4.2.5 ESTABLISHMENT OF FISHERIES
4.2.5.1 Fish introduction.
Fishes may accidentally enter a pit lake during flooding if the source water contains fish.
For example, Lac Des Roche, a former open pit mine in Alberta, was inhabited by several
fish species during rapid filling by nearby West Jarvis Creek (see Schwartz, 2002, and
several references therein). Nearly twenty years after initial filling, the lake sustains a
recreational fishery (Schwartz, 2002). Fish can also be introduced into a pit lake after
flooding if there is a surface connection to a nearby, fish-bearing water body. For
example, the open pit at the Gunnar uranium mine was flooded with water from nearby
Lake Athabasca (Tones, 1982). Although the channel connecting the two water bodies
had been closed for nearly 20 years at the time of the investigation, Tones (1982) found
four fish species, including northern pike, in the pit lake. All of these species presumably
dispersed from Lake Athabasca when there was a channel between the two lakes,
allowing for floral and faunal exchange.
Fishes can also be introduced into pit lakes by human transplantation or supplementation
(stocking), usually for recreational or aquaculture purposes, such as in East Pit Lake in
central Alberta. This lake was created from the remediation and the reclamation of a
former surface coal mine near the town of Wabamun, Alberta (Sumer et al., 1995).
Subsequent to filling the pit with water, the newly created East Pit Lake was stocked with
rainbow trout (Oncorhynchus mykiss; Sumer et al., 1995). Although this lake is
essentially a put-and-take fishery, and is continually stocked, East Pit Lake continues to
sustain a quality recreational fishery for rainbow trout. Fish may also be introduced to
lakes for aquacultural purposes. In Minnesota, for instance, hundreds of abandoned iron
ore pits have filled with groundwater and surface water to create lakes (Yokom et al.,
1997) and a handful of these lakes have been reclaimed for the purpose of salmon
aquaculture (Axler et al., 1998). These lakes may be more suitable for aquacultural
purposes in comparison to natural lakes since they usually have no connection to any
other waterbody, thereby eliminating the risk of fish escaping which could have drastic

60
impacts on the aquatic ecosystem (Naylor et al., 2005). Extensive aquaculture could have
severe impacts on the water quality of the lake, such as eutrophication (Yokom et al.,
1997; Axler et al., 1998).
4.2.5.2. Fisheries habitat creation and enhancement.
Relative to natural lakes, the structural habitat complexity and diversity found in pit lakes
is considerably reduced. For instance, in artificially created lakes, there may be minimal
or no structural habitat important to fishes such aquatic vegetation and course woody
debris. Therefore, habitat creation, enhancement, or rehabilitation should be an integral
part of the initial reclamation process during mine decommissioning; especially if
fisheries are to be established. Figure 23 provides a general summary of fisheries habitat
creation and enhancement for pit lake water bodies.
Figure 23. Examples of fish habitat creation and enhancement techniques as described in
Section 4.2.5.2. Artificial reef created with rubble and waste-rock (A), floating reef-raft
(B), large boulders for cover (C), the planting of littoral (D) and riparian vegetation (E),
submerged conifers for providing cover (F), construction material such as donated
concrete (G) and the addition of large woody debris (H).
Habitat creation and enhancement in pit lakes may be more easily facilitated, in
comparison to natural lakes, since most of the reclamation activities can occur when the
Open Mine Pit
E
D
C
B
A
D
E
F
A
G
A
H
G
A
Open Mine Pit
E
D
C
B
A
D
E
F
A
G
A
H
G
A
Open Mine PitOpen Mine Pit
E
D
C
B
A
D
E
F
A
G
A
H
G
A
61
mine pit is free of water. Additionally, in many cases, pit lakes will be larger than the
original waterbody thereby allowing for the creation of more fish habitat compared to
that which was originally present (BHP, 1999). The ultimate goal for fish habitat
restoration is to provide habitat that is specific to the target fish species for all life history
stages, and habitat that will allow for the establishment of, or provide refuge for, their
food source (e.g., aquatic invertebrates). In northern environments, overwintering habitat
is also critical, but likely is not a limiting factor in pit lakes given the abundance of
deepwater habitat that is usually present in these waterbodies (BHP, 1999). Subsequent to
initial reclamation practices, pits will be filled with water, either naturally or from nearby
water bodies. If filled directly from a nearby water body, or if viable stream connections
are re-established, fish communities may establish naturally via the transfer of native fish.
If not, fish species will have to be supplemented or re-stocked into the waterbody.
Regardless of the method used, suitable fish habitat is a perquisite to the persistence of
fishes in these new waterbodies.
Regarding newly created pit lakes, the riparian zone - the interface between the terrestrial
and aquatic environments - will likely be deficient in structural habitat and riparian
vegetation. Typically this zone provides many ecological functions important to
surrounding waterbodies. For example, autochthonous inputs from riparian vegetation
such as leaves and terrestrial invertebrates are an important food source for shredding
invertebrates and fishes respectively. As such, it is important to determine appropriate
reclamation strategies for the creation and enhancement of riparian habitats. Often,
because of their physical profiles, large-scale landscaping is needed to facilitate in the
alteration of the mine profile (see Section 4.2.1). Once completed, if landscaping is
required, replanting or establishing native vegetation in riparian areas is likely the most
effective way of rehabilitating, or creating, productive lake shore areas. This in turn will
promote bank stability and if trees can be successfully established, large woody debris
may be contributed to the lake over time, thereby increasing structural complexity of near
shore environments. Native plant and tree species used in riparian plant establishment can
either be transplanted from other nearby donor sites (e.g., Vincent, 1995), or planted from
seed (e.g., Reid and Naeth, 2005a, b). Regardless of the method used, preference should
be given to species that can withstand more rigorous conditions. Once vegetation is
established, more environmentally sensitive species may be introduced (Vincent, 1995).
Given the steep-sided profile (sometimes 1:1 slopes) of most pit mines, critical littoral
habitat is usually minimal or even completely absent in pit lakes. Shallow water may
exist in localized areas, such as mine benches or haul roads (Tones, 1982). This has
major ecological implications given that the majority of northern fish species will, at one
time or another during their life cycle, be reliant on the littoral zone or the habitat
structures within that area for spawning, feeding, or finding refuge. Shallow water habitat
may be artificially created in a pit lake by bulldozing, drilling or blasting the upper walls
onto the upper bench of the pit, partial backfilling with mine waste, dumping blast rock
into the lake from shore or from ice cover, or manually constructing bays around the
periphery of the pit (BHP, 1999). Following completion of these larger scale
modifications the addition of smaller substrate features such as crushed rock, gravel, or
materials of smaller sizes to fill some of the interstitial spaces will be important for
62
benthos establishment, littoral macrophyte rooting, and for providing appropriate
spawning substrates.
Artificially created littoral areas will need structural additions or modifications in order to
increase the complexity of these habitats. Waste rock and/or excavated rock can be piled
in nearshore habitats to create artificial reefs (BHP, 1999) that will increase interstitial
refuge for juveniles and forage fish, create vertical relief or can be used for spawning by
some species. Rock-rubble has been used to successfully create lake trout spawning
habitat (Foster and Kennedy, 1995; Moy, 1995), and cover for fry and young-of-the year
fish (Fitzsimons, 1996). Additionally, scrap concrete or stone rubble that may be present
as a result of mine decommissioning can be used to increase structural habitat (Kelch and
Reutter, 1995). In some cases, these artificial structures are used more by fishes than
natural ones (Fitzsimons, 1996). The size of spawning substrates should be consistent
with the needs of the target species to be stocked into the lake. For example, lake trout
and arctic grayling, two candidate fish species for pit lakes in northern Canada, differ in
their preferred spawning substrates (Scott and Crossman, 1998). Lake trout prefer to
spawn over large boulder or rubble whereas Arctic grayling prefer to spawn over gravel-
dominated substrates (Scott and Crossman, 1998). If the creation of artificial reefs is not
practical, the creation of wooden reef-rafts may be more suitable. These artificially
created rafts can be covered with a variety of material (earth, pebbles, grass, woody
debris), anchored to the lake bottom and will provide cover for fish and nesting sites for
aquatic birds (Blokpoel, 1995). The disadvantage of these structures is that they may
have to be removed every year before freeze-up to avoid damage (Blokpoel, 1995).
Artificial “floating islands” have recently been used to improve water quality and
enhance wildlife habitat in ponds and wetlands polluted by agricultural or urban runoff
(Headley and Tanner, 2006; Stewart et al., 2008). The patented BioHaven© product is
one example, and is essentially a moored platform constructed of recycled plastic
containers which is then covered with sod and seeded with wetland plants. Case studies
summarized by Headley and Tanner (2006) and on the internet (FII, 2008) document
significant improvements in water quality where floating islands have been deployed,
including a reduction in nutrient concentration and a decrease in turbidity. The islands
also provide excellent habitat for macroinvertebrates, fish, waterfowl, and aquatic
mammals. Although the authors are not aware of a case study where floating islands
have been deployed in a mining pit lake, this would seem to be an interesting opportunity
for future research.
In addition to artificial reefs, the addition of woody debris will be an important
component of pit lake habitat restoration. Woody debris is most often used in stream
habitat restoration (e.g., Cederholm et al., 1997). When used in lakes, woody debris
increases structural habitat complexity and diversity and is mainly used to provide cover
for juvenile and forage fishes (Roni et al., 2005; Roth et al., 2007), which are
subsequently consumed by piscivorous fishes. Additionally, successful colonization and
growth of aquatic invertebrates will likely depend on the establishment of suitable habitat
features such as those provided by woody debris (Jones et al., 2003), which will in turn
provide an important food source for non-piscivorous or juvenile fishes. Structures such
63
as log piles, submerged logs, woody brush piles, or even bundles of conifers (e.g., black
spruce, Picea mariana) native to the area and brush/tire bundles can be created and
situated at varying depths throughout littoral areas of the lake. Several studies have
shown that such artificially created structures of woody debris will often lead to an
increase in fish diversity and abundance of fishes (Smokorowski et al., 1998, 2006, and
references therein). For example, Koski (1992) showed that the density of parr of coho
salmon (Oncorhynchus kisutch) increased significantly in streams with increasing volume
of large woody debris. In a lacustrine setting, Newbrey et al. (2005) reported that fish
abundance always increased in the presence of coarse woody debris, and that habitat
provided by coniferous trees was more important than that provided by deciduous trees.
Consequently, the role of woody debris as an important component in littoral habitat
complexity must be taken into consideration in the creation and enhancement of critical
fish habitat, specifically within littoral areas.
Littoral aquatic macrophytes are important contributors to fish habitat and increase
shoreline diversity for aquatic organisms, and hence are important in the creation and
enhancement of productive fish habitats in newly created pit lakes. Similar to woody
debris, aquatic macrophytes provide cover for forage and juvenile fish, provide ambush
cover for piscivorous fish, and will allow for the establishment and persistence of aquatic
invertebrates. Aquatic vegetation and organic matter is an important food source for
aquatic invertebrates, and the lack of this vegetation may result in a decrease in
abundance of these organisms (Jones et al., 2003). Additionally, aquatic vegetation will
also increase dissolved oxygen levels and, depending on the species, provide suitable
spawning habitat. Several species of fish, including some species common to northern
environments, require aquatic vegetation directly for spawning whether for adherence of
eggs, or the building of nests (e.g., northern pike, yellow perch Perca flavescens and
ninespine stickleback Pungitius pungitius; Scott and Crossman 1998). As such, it is
important that both submergent and emergent aquatic vegetation, in addition to shoreline
vegetation such as sedges and reeds, be included in reclamation practices to create,
restore or enhance fisheries habitat in the littoral zones of newly created lakes. Aquatic
vegetation can be collected from nearby donor sites (Grillmayer, 1995; Vincent, 1995), or
possibly planted from seed, although no examples of this could be found (see Reid and
Naeth, 2005b for a terrestrial example), therefore collection from donor sites may be
more plausible. If donor plants from a nearby waterbody are used, tubers or whole plants
can be relocated to the new site (e.g., Grillmayer, 1995). Plants should be transferred in
soil, kept moist and with as much of the root as possible to increase the chance of
establishment (e.g., Vincent, 1995). Potential emergent and submerged donor species
typically found in littoral aquatic environments of the Northwest Territories include
cattails Typha lattifolia, pondweed, Potamogeton angustifolium, and P. minimum, yellow
pond lily Nuphar variegatum, and water milfoil Myriophyllum exalbescens, and M.
verticillatum. Important shoreline vegetation might include a variety of sedges Carex
spp., cattails and rushes Scirpis spp. In general, the plant species used should be one
common to the area of the newly created pit lake and preferably a rigorous pioneer
species able to establish in areas of recent disturbance.

64
Several other factors must be taken into consideration with respect to enhancing the
quality and quantity of pit lake fish habitat. For example, as discussed in detail in sections
4.2.3 and 4.2.4, biological or chemical treatment of the water may be necessary to
improve water quality to ensure that it is non-lethal and/or non-harmful for the entire
biological community of the lake including zooplankton, phytoplankton, invertebrates,
macrophytes, fish and wildlife. To promote the bacterial community of the newly created
lake, it may be necessary to transport natural sediment from nearby lakes in order to
innoculate benthic bacteria in these systems. Burlap sacks can be used as they are
durable, porous, and biodegradable (H. Larratt personal communication, Larratt Aquatic
Consulting Ltd).
Additionally, if stream courses have been altered, and upon mine
decommissioning are being diverted back into the pit, stream habitat restoration may also
be necessary. If so, most of the habitat creation and enhancement ideas mentioned above
can also be applied to lotic systems as well. Lastly, if the end use of the pit lake is strictly
for aquacultural purposes, then it may not be necessary to create and enhance habitat to
the degree described since these fish will be artificially fed and often protected from
predation.
4.2.5.3. Potential fish species.
If fish need to be transplanted from another water body, with intent of establishing a self-
sustaining ecosystem, or even a put-and-take fishery, several important things must be
considered. First, if the lake is connected to other water bodies in the area, only native
fish species should be used. The negative impacts of the introduction of non-native fish
have been reviewed extensively in the literature (e.g., Taylor, 2004). Ultimately, the
introduction of non-native species can result in local extinctions of native species via
competition, predation, disease transmission, habitat modifications or in some cases
hybridization. Second, to promote self-sustaining fisheries and/or aquatic ecosystems a
conscious effort must be made to attempt to mimic the composition and diversity of
species in other lakes in the area. Replicating species composition increases the chances
of establishing fisheries by fulfilling all trophic levels and ecological niches. Lastly, fish
to be transplanted in the new waterbody should be collected from a disease free source
for obvious reasons. For example Ogawa et al. (2004) discuss the dangers of
translocating cyprinid fish infected with metacercariae trematodes, and how even one fish
translocation could be devastating to an aquatic ecosystem.
Taking the above into consideration there are several fish species (both priscavors and
forages fishes) in northern environments that can be considered suitable candidates for
translocation, although this will have to be evaluated on a site specific basis. In the
Northwest Territories for example, the list may include, but is not limited to burbot (Lota
lota), northern pike, lake trout, arctic char, lake whitefish (Coregonus clupeaformis), least
cisco (C. sardinella), arctic cisco (C. autumnalis), lake cisco (C. artedii) long nose sucker
(Catostomus catostomus), white sucker (C. commersonii), walleye (Sander vitreus) and
yellow perch, slimy sculpin (Cottus cognatus), spoonhead sculpin (C. ricei), brook
stickleback (Pungitius pungitius), lake chub (Couesius plumbeus), pearl dace
(Margariscus margarita), and long nose dace (Rhinichthys cataractae; Evans et al.,
2002).

65
5. CASE STUDIES AND EXAMPLES
5.1 KIMBERLITE DIAMOND MINES
The rapid growth of diamond mining in northern Canada was a major impetus for this
report. The following discussion gives more information on the geology, hydrogeology,
and geochemistry of kimberlites and their associated groundwaters.
5.1.1 Kimberlite geology
Kimberlite is an altered ultramafic potassic igneous rock that originates in the earth’s
mantle at or near the base of the lithosphere (McBirney, 1993; Klein and Hurlbut, 1993).
It has a brecciated or porphyritic texture, with relatively large megacrysts in a fine-
grained groundmass. The groundmass contains minerals that are typically too small to
see with the naked eye, including calcite, serpentine, and clay. The megacrysts include
diamond, olivine, serpentine, phlogopite, ilmenite, pink garnet, bright green Cr-diopside,
apatite, spinel, perovskite, enstatite, and/or monticellite (Klein and Hurlbut, 1993).
Kimberlite deposits (Fig. 24) typically occur as steep-sided cylindrical “pipes” that are
filled with a mixture of kimberlite and country rock fragments (xenoliths). The
kimberlite itself can have a coherent igneous texture (often referred to as the “hypabyssal
facies”), or a fragmental texture (often referred to as the “pyroclastic” or “crater facies”).
These two facies broadly define portions of the kimberlite that are intrusive (plutonic) or
extrusive (volcanic) in origin, respectively. Pyroclastic kimberlite typically fills the top
of the pipe and can drape over the crater rim to form a tuff-like volcanic layer.
Kimberlites may form a crater lake at their surface, as evidenced by sedimentary re-
working of the pyroclastic facies.
Figure 24. Simplified geology of a kimberlite pipe.
hypabyssal
facies
kimberlite
country
rock
pyroclastic
facies
kimberlite
xenoliths

66
5.1.2. Kimberlite hydrogeology
Based on limited data, Morton and Müller (2003) obtained hydraulic conductivity (K)
values of 2 x 10
-5
m/day (a rather low value) to 0.4 m/day (quite high) for kimberlite at
the Venetia diamond deposit, South Africa. This very large range in K may reflect
primary geological heterogeneity (e.g., different kimberlite facies), differences in the
degree of weathering, and also, importantly, the extent of secondary fracturing during
blasting and excavation. The surrounding fractured country rock at Venetia had a range
in K of 2 x 10
-5
to 1 x 10
-3
m/day. Morton and Müller (2003) include a table of published
K values from other South African kimberlite deposits, and these vary in the range 2 x
10
-5
to 0.04 m/day. In general, higher K values were reported for “sedimentary”
kimberlite (equivalent to pyroclastic facies of this report) and lower K values for
hypabyssal kimberlite. In a study of the hydrogeology of the Diavik mine in NWT,
Kuchling et al. (2000) measured average K values of 0.017 to 0.043 m/day for kimberlite
and country rock. Overlying lakebed sediments and till at Diavik had an average K value
of 4 x 10
-3
cm/sec (3.4 m/day). The rather high K values for kimberlite at Diavik may
reflect the predominance of pyroclastic facies at this mine.
Kuchling et al. (2000) give a good description of the distribution of permafrost near
Diavik. In this region, the total depth of penetration of the permafrost layer varies over a
huge range, being as much as 350m deep below large land masses, to being nonexistent
below most lakes with an average depth exceeding the depth of maximum winter ice
cover (~ 1.5 to 2 m). The distribution of permafrost around a mine site is a key variable,
as permafrost is essentially impermeable to water transport. Kimberlite deposits such as
Diavik that are located near a lake would be expected to produce copious amounts of
groundwater during mining which will need to be managed appropriately. This also
means that such deposits have a good chance of filling rapidly upon closure, although this
may depend on whether or not permafrost re-establishes itself on the exposed mine walls
during the mine life.
Most open pit kimberlite mines are steep-sided and roughly circular in outline (e.g., see
Figs. 1, 20). Related to this morphometry, Morton and Müller (2003) described
concentric faults and fractures in a so-called “zone of relaxation” (ZOR) that opened up
in the mine walls surrounding the Venetia open pit. These extensional cracks outcropped
at the surface, and also penetrated to significant depths before attenuating. Morton and
Müller (2003) point out that the concentric fractures act as corridors for enhanced
groundwater flow, and that care should be taken so as not to locate mining facilities
within the ZOR. For example, vertical cracks beneath a tailings pond could promote
downwards infiltration of tailings water towards the pit. Given the similar shapes of
diamond mines worldwide, it is plausible that concentric fractures of this type could
develop elsewhere, although this will be depend on the mechanical properties of the
country rock at each individual mine, which will vary widely from site to site.

67
5.1.3. Chemistry of groundwater associated with kimberlite deposits
A literature review uncovered only a few investigations of the chemistry of groundwater
associated with kimberlite deposits, none of which dealt specifically with pit lakes.
From an acid-base accounting perspective, most kimberlite deposits appear to have low
AP and moderate to very high NP, meaning that they have a low to very low potential to
generate acidic leachate. In a recent study of kimberlite ore at the Ekati mine, NWT,
Rollo and Jamieson (2006) obtained average NP and AP values of 240 and 13 kg
CaCO
3
/ton, respectively, for an NP/AP ratio of 18. Similar values were reported for the
Diavik mine, NWT (Baker et al., 2003). Kimberlite itself typically has a very low sulfide
mineral content, although pyrite and other sulfides may be present in the surrounding
country rock, or in xenoliths within the pipe itself. For example, Baker et al. (2003)
found that mudstone xenoliths in the Diavik kimberlites contained up to 1% pyrite, and
could be a potential source of acidic leachate in the event that the xenoliths were isolated
by mining or milling activity from the surrounding kimberlite rock. Rollo and Jamieson
(2006) also pointed to country rock xenoliths as a potential source of water
contamination, and suspected that leaching of sulfate minerals (anhydrite, gypsum) from
the country rock fragments may have been a major factor in increasing the TDS of
groundwater near the mine site.
Given the high NP/AP ratio and presence of carbonate minerals in the altered
groundmass of most kimberlites, pH values near or above neutrality would be expected.
Rollo and Jamieson (2006) reported pH’s of 7 to 8.5 for pore waters in contact with
kimberlite at the Ekati mine. Interestingly, Sader et al. (2007) found that groundwater
associated with unmined kimberlite deposits in northeastern Ontario had pH values in the
range 8.5 to 12.4, with roughly half of the waters sampled having pH > 10. These
remarkably high pH values were attributed to inorganic chemical reactions taking place
during interaction of groundwater with olivine and serpentine. Worldwide, strongly
alkaline groundwater is known to be associated with areas of the Earth where low
temperature serpentinization of ultramafic rocks is occurring (Barnes and O’Neil, 1969).
Kimberlite groundwater also appears to be enriched in Cl and F relative to regional
groundwater, which may be due to leaching of halide elements from phlogopite, apatite,
serpentine, and other hydrous minerals in the kimberlite pipe. The unusual chemistry of
waters associated with kimberlite led Sader et al. (2007) to propose that groundwater
sampling could be an important exploration tool for undiscovered diamond deposits.
Sader et al. (2007) found elevated partial pressures of hydrogen (H
2
) and methane (CH
4
)
gas in many of their kimberlite-hosted groundwater samples, and also report some of the
lowest Eh values ever published for natural water samples (down to -500 mV). Based on
stable isotope trends, the authors concluded that the methane was biogenic in origin.
In terms of TDS, Morton and Müller (2003) indicate that some of the groundwater
samples at the Venetia mine were moderately to highly saline, with Na
+
and Cl
-
being the
dominant ions. Day et al. (2003) and Rollo and Jamieson (2006) also reported high
salinity for pore waters associated with kimberlite at the Ekati mine, with Ca
2+
and Mg
2+
being the dominant cations and sulfate being the dominant anion. In contrast, most of the
groundwater examined by Sader et al. (2007) had low to moderate salinity. It is difficult
to generalize from these observations. In the case of Venetia, some of the high TDS

68
groundwaters could be an artifact of evapo-concentration in the hot, dry climate (as
suggested by Morton and Müller, 2003), whereas in the case of Ekati, the high TDS could
reflect leaching of chemically unstable minerals from kimberlite and/or country rock
xenoliths (as suggested by Rollo and Jamieson, 2006). Day et al. (2003) also pointed out
that the TDS of some of the water at the Ekati mine site could have increased through
freeze concentration (i.e., brine exclusion during freezing of water). This process is
known to increase the concentration of solutes in pore water and shallow groundwater in
the active layer of permafrost regions (Konrad and McGammon, 1990; Lacelle et al.,
2007).
Day et al. (2003), Rollo and Jamieson (2006), and Sader et al. (2007) include tables of
chemical analyses of trace elements in waters associated with kimberlite. From the
combined results, it appears that kimberlite waters should have low concentrations of
most metals and metalloids of concern, such as As, Cd, Co, Cu, Cr, Pb, Se, and Zn.
Exceptions include Fe (up to several mg/L in some samples reported by Day et al., 2003),
Ni (average of 150 µg/L in the study of Rollo and Jamieson, 2006) and Al (up to 0.5
mg/L in some of the groundwaters sampled by Sader et al., 2007). Given the generally
high pH of kimberlite waters, elevated Fe concentrations would only be expected under
anaerobic conditions where Fe is soluble as Fe
2+
. Oxidation of Fe-rich water could result
in locally acidic conditions, as was described by Day et al. (2003) for some small acidic
seeps and pools near the toe of a kimberlite containment facility at Ekati. Dissolved Al
concentrations in the 0.1 to 0.5 mg/L range are known to be harmful to many species of
fish (Driscoll et al., 1980; Baker and Schofield, 1982). However, because kimberlite
waters are also characterized by a high fluoride (F
-
) content (Sader et al., 2007), it is
likely that any dissolved Al will be strongly complexed with F
-
, thus lowering the
bioavailability of the metal (Driscoll et al., 1980).
A quick review of the literature suggests that high salinity may be a feature that is
common to deep groundwater in northern, permafrost regions. Kuchling et al. (2000)
found a regional trend of increasing TDS with depth from a number of wells in the Lac
de Gras region. Groundwater samples collected at depths of up to 500m (i.e., the inferred
range for most existing or planned mining operations) generally had less than 1000 mg/L
TDS, although some exceptions exist. In contrast, many of the groundwater samples at
depths greater than 1000m had TDS > 10,000 mg/L. Kuchling et al. (2000) pointed out
the possibility that a deep mine could encounter upwelling, highly saline groundwater.
Although we know of no example where this has happened for a Canadian diamond
mine, very high salinity groundwater has been reported from diamond mines in northern
Russia (Borisov et al., 1995; Alexeev and Alexeeva, 2003).
5.1.4. Implications to diamond mine pit lakes in northern Canada
Based on the preceding discussion, a number of conclusions can be drawn that have
implications to end uses of diamond mine pit lakes in northern Canada. These ideas
range from speculative to probable, and will likely vary in applicability from site to site.
69
•
In the event that a mine is located some distance from a major body of water,
thick permafrost (up to 350 m) is likely to be encountered. If the mine penetrates
below the permafrost zone, there is the possibility of upwelling groundwater that
could be moderately to highly saline. Such a situation would be favorable for
development of a meromictic pit lake upon closure.
•
In the event that a mine is located beneath or along the shoreline of a lake,
permafrost is likely to be thin or absent. Water management will be a major
factor during and after the period of active mining. Groundwater entering the
mine will likely have a chemistry that is similar to that of the nearby surface water
body, or will be a mixture of deeper groundwater and lake water.
•
The hydraulic conductivity of bedrock within and immediately adjacent to a
kimberlite open pit is likely to be highly variable, but will probably include at
least some zones of high permeability along fractures and faults. Overall,
permeability should decrease with depth in the mine.
•
Groundwater associated with the kimberlite rock itself is unlikely to pose major
problems from a water quality point of view, although the possibility exists of
locally high TDS and/or high pH. The possibility of pH > 10 exists for deep,
undiluted pore waters in contact with kimberlite. Such high pH values are
probably unlikely for an entire pit lake, especially for the epilimnion where free
exchange of CO
2
exists between air and the water column.
•
Based on limited data from the literature, water associated with kimberlite rock
should have very low concentrations of trace metals, with the possible exceptions
of Fe, Ni, and Al.
•
Although the kimberlite itself may pose few water quality problems, no general
statement can be made about the surrounding country rock. As shown at both
Ekati and Diavik, country rock xenoliths have a much higher potential to generate
acidic or metal-rich leachate than the kimberlite bodies themselves. Because the
geology of the country rock at each mine may be completely different, the
environmental aspects of diamond mining will need to be evaluated on a case-by-
case basis.
5.2 COLOMAC GOLD MINE, NORTHWEST TERRITORIES
The Colomac Mine is an interesting example of a mining pit lake at high latitudes which
was remediated to reduce concentrations of cyanide and its by-products. Most of the
following information stems from a presentation by Chapman et al. (2007) and a follow-
up memorandum (SRK, 2007). Additional details on the limnology of this site appear in
several publications by Pieters and Lawrence (e.g., Pieters and Lawrence, 2007).
The Colomac Mine operated between 1990 and 1997 and produced 16.7 tonnes of gold
using a cyanide vat leaching process. Tailings wastes containing cyanide-rich water were
discharged to two nearby lakes, one of which was completely filled with solids, and the
other – the so-called Tailings Lake – was only partly filled. To prevent the discharge of
70
cyanide-rich water from Tailings Lake, part of the lake was diverted into the nearby Zone
2 open pit, forming a pit lake. The Zone 2 lake is about 50% comprised of Tailings Lake
water, the other half being natural groundwater inputs as well as rain and snowmelt.
The main contaminants of concern at Colomac include free cyanide (CN
-
or HCN),
complexed cyanide (e.g., Fe(CN)
6
2-
), thiocyanate (SCN
-
), ammonium (NH
4
+
), and nitrate
(NO
3
-
). Whereas cyanide and thiocyanate are residual compounds left over from the
metallurgical recovery of gold, ammonium and nitrate are products of the breakdown of
cyanide and thiocyanate. These reactions can be written as follows (modified from
Chapman et al., 2007):
CN
-
+ ½O
2
+ H
+
+ 2H
2
O → HCO
3
-
+ NH
4
+
(32)
SCN
-
+ 2H
2
O + 2O
2
+ H
2
O → SO
4
2-
+ HCO
3
-
+ NH
4
+
+ H
+
(33)
NH
4
+
+ 2O
2
→ NO
3
-
+ 2H
+
+ H
2
O
(34)
Because all of these reactions consume oxygen, low concentrations of DO were also an
issue, especially for the much deeper Zone 2 pit lake.
Early monitoring showed that both free and complexed cyanide in both lakes broke down
rapidly after closure to values that met discharge requirements, presumably from a
combination of oxidation and biological uptake. However, thiocyanate concentrations
remained elevated. To enhance the breakdown of SCN
-
, a decision was made to add
mono-ammonium phosphate (NH
4
H
2
PO
4
) to both lakes to stimulate growth of algae and
thereby promote Enhanced Natural Removal (ENR). The P-amendments took place in
2002-2003. The results showed rapid and quantitative removal of thiocyanate from the
relatively shallow Tailings Lake, but less complete removal from the Zone 2 pit lake.
The main reason for the lower removal efficiency in the latter case was that the much
deeper pit lake was meromictic during this time period so that thiocyanate was eliminated
only in the upper 20m of the lake. To get around this problem, the operators constructed
an aeration circuit in which compressed air was delivered to the deep lake to promote
oxidation and to destratify the water column. Aeration was conducted in 2006 and 2007,
and results indicate that not only was destratification successful, but also that thiocyanate
and ammonia have been essentially removed from the entire Zone 2 pit lake (SRK, 2007).
Although the combination of ENR and aeration have been successful for eliminating
cyanide, ammonia, and thiocyanate from the Colomac lakes, there are lingering concerns
over elevated phosphate and nitrate concentrations. Fortunately, there is evidence that
significant bacterial reduction of nitrate to N
2
gas (reaction 20) occurs in the Zone 2 pit
lake during the frozen winter months (SRK, 2007). Projected to the future, it is possible
that most of the nitrate in the Zone 2 lake will be gone in 3 to 5 winter seasons (SRK,
2007). However, this assumes that the water column will continue to become anoxic
during the winter months, a prerequisite for denitrification to occur (see section 2.4.2.8).
Seasonal anoxia may not occur now that the major sources of chemical oxygen demand
(ammonia, cyanide, thiocyanate) have been removed. The removal of P also presents a
challenge, although the long-term trends in monitoring data show a steady decline in total
P load with in the Zone 2 pit lake (SRK, 2007).
71
5.3. EAST PIT COAL MINE, ALBERTA
Alberta has a long history of coal mining operations which have played an important role
in the early development and colonization of the province. In the foothills and mountains
of the province, mining for coal has been proceeding since the turn of the nineteenth
century and the landscape in such areas has been altered drastically. Often as surface
mining operations proceed and/or subsequent to mineral extraction the mined-out pits are
backfilled with overburden, and where the pits cannot be filled completely an open pit
remains. Eventually, after dewatering of the mine pit ceases, groundwater and surface
water fills the pit resulting in an “end pit lake”, which is typically proposed as a
reclamation technique for an end-use for such pits upon mine closure (Alberta
Environment, 2004).
The resultant lakes vary in morphometry depending on the shape of the coal seam and the
type of mining method used. For example, dragline mining typically results in long,
narrow, deep lakes (usually < 30m) relative to their width whereas truck and shovel
operations usually create larger more angular lakes that can be much deeper (up to >
70m) (Alberta Environment, 2004). Additionally, the shape and size of the newly created
lakes will be highly influenced by the placement of the overburden used in backfilling
operations and the shape of the coal seam that was mined. In Alberta, several examples
exist where lakes have been created in decommissioned surface coal mines during the
reclamation process.
East Pit Lake, near the town of Wabamun, Alberta, is a specific example of a pit created
by dragline mining that has been reclaimed into a lake that currently supports a
productive recreational fishery, and provides an array of other recreational opportunities.
After mining, the East Pit was filled with groundwater to replace two small water bodies
that had to be drained to access the coal (Sumer et al., 1995). Currently the lake is
approximately 100m wide, 800m long with an average depth of 3.3m and a maximum
depth of 10m (Sumer et al., 1995; Figure 25).
Prior to filling with groundwater, physical contouring of the final cut of the coal mine pit
was required to meet the reclamation goals of creating a put-and-take fishery, and
enhancing the surrounding area to be used for recreational activities such as picnicking
and bird-watching (Sumer et al., 1995). Once water was drained from the original pit,
major earthwork was completed using a dragline while finishing work was facilitated
with the use of bulldozers where, in total, 1.82 million m
3
was moved during construction
(Sumer et al., 1995). This was done to increase littoral habitat and to ensure adequate
depths for fish survival. In addition to physical contouring, much of the reclamation
strategy focused on the area surrounding the lake. For example, grass, trees and shrubs
were planted, and fertilizer was applied to the surrounding area to promote revegetation
of native plant species which would promote bank stability, provide a food source and
cover for native animals, and promote the input of terrestrial insects into the lake.

72
Figure 25. East Pit Lake, Alberta was formed through the reclamation of a surface pit left
over from a dragline coal mining operation.
As discussed in several places throughout this document, the weathering of newly
exposed material in a mining environment can accelerate the release of acid and metals
into newly created pit lakes. No water quality data were found for East Pit Lake during
the preparation of this report other than the fact that East Pit Lake has similar DO
concentrations to natural lakes in the area (Sumer et al., 1995). Given the successful
establishment of aquatic organisms in this waterbody (Sumer et al., 1995), it is likely that
other water quality parameters – such as pH, salinity, and metal concentrations – are also
good.
Subsequent to groundwater filling, shoreline habitat creation and enhancement
procedures commenced. For instance, logs and brush were submerged along the
shoreline, and brush piles were placed at varying depths to provide cover in deeper parts
of the lake (Sumer et al., 1995). Additionally, submergent and emergent aquatic
vegetation, such as Richardson’s pondweed (Potamogeton richardsonii) and northern
watermilfoil (Myriophyllum exalbescens) were established in the lake (Sumer et al.,
1995), and both are recognized as important fish habitat (e.g., Copp, 1997). This aquatic
vegetation will provide refuge for aquatic invertebrates that are valuable food for stocked
fishes and furthermore will increase dissolved oxygen within the waterbody.
73
As a combined result of the major earthwork and habitat modifications, East Pit Lake
currently harbors a diverse community of aquatic plants, aquatic invertebrates, and fish
(Sumer et al., 1995). Additionally, the lake provides excellent recreational fishing
opportunities for rainbow trout (pers. observation), and provides users with opportunities
for bird watching, hiking and picnicking. TransAlta Utilities, the company at the
forefront of this habitat reclamation and creation project, has since received numerous
awards for their efforts in establishing this well known sport fishery.
5.4 IRON ORE MINES, MINNESOTA AND ONTARIO
The Portsmouth Mine Pit Lake in Minnesota is a good example of a very large (Fig. 2)
man-made lake formed after flooding of an iron ore (hematite) mine. This is one of the
deepest lakes in Minnesota at 137 m, and is one of the largest of > 200 pit lakes in the
region left behind from historical iron mining. No technical papers could be found on the
chemistry or limnology of this lake. However, the lake apparently has excellent water
quality as it is stocked with rainbow trout and – according to numerous websites,
including Wikipedia – is used extensively for recreation, including swimming, boating,
and scuba diving (Fig. 26). A recent fisheries survey indicated a trout density of 56
individuals per hectare, which is similar to natural lakes in Minnesota (Close et al., 2006).
This relatively high fish density is somewhat surprising, considering that the lake is also
reported to be essentially devoid of littoral habitat (Close et al., 2006). Two other large
mining pit lakes in the same region with 13-14% littoral habitat had slightly lower fish
densities than Portsmouth Lake (Close et al., 2006). However, these observations should
be interpreted with caution since all of these lakes are stocked and therefore trout
populations are artificially manipulated. As mentioned above, a number of pit lakes in
Minnesota from former iron mines were used for large fish farms in the early 1990s, but
much of this activity has ceased due to unwanted eutrophication of the lakes from
aquaculture activities (Axler et al., 1996, 1998).

74
Figure 26. Photograph of the swimming beach at the Portsmouth Mine Pit Lake,
Minnesota (taken from Wikipedia.org).
Mining of similar iron ore deposits in Ontario has also resulted in pit lakes, as well as
dammed reservoirs. The Steep Rock mines of NW Ontario (near Atikokan) are one
example where several large natural lakes were completely drained and buried with waste
rock and overburden during iron ore mining operations (Sowa, 2004). Following closure
of the mines, several of the mine pits were flooded and dams were built to raise the stage
of the impounded water, creating a new reservoir/pit lake system that supports a
population of bass (Micropterus spp.), walleye (Sander vitreus), northern pike (Esox
lucius), lake whitefish (Coregonus clupeaformis), and cisco (Coregonus artedi). A large
floating fish farm operation has also been established (McNaughton et al., 1999; Sowa,
2004).
A recent abstract (Godwin et al., 2007) reported possible water quality problems at Steep
Rock associated with the anticipated release of water from two newly flooded mine pit
lakes that are connected to the main lake system. The main contaminant of concern
appears to be sulfate, with reported values of 1200-2000 mg/L SO
4
in one of the pit lakes
that will be discharging to surface water. Godwin et al. (2007) state that one of the
concerns is the health of aquatic plants such as duckweed (Lemna minor), which
reportedly are impacted by sulfate concentrations as low as 1000 mg/L.
5.5 SLEEPER GOLD MINE, NEVADA
The Sleeper gold mine of NW Nevada was operated for roughly 10 years, from the mid-
1980s to the mid-1990s. The history of closure and flooding of this large open pit mine
75
(see Fig. 2) is an interesting case study (Dowling et al., 2004). During the period of
active mining, a very prolific groundwater aquifer overlying the ore body was
intercepted. Dewatering wells were pumped at rates up to 930 L/s, making this one of the
largest dewatering operations worldwide. Meanwhile, geochemical studies and ABA
tests performed during the mine life showed that a high percentage of the lower mine
walls and excavated waste rock had a moderate to high potential to generate acidic and
metal-rich leachate.
As described by Dowling et al. (2004), several steps were taken to mitigate the potential
for AMD at this site. To begin with, the open pit was partially backfilled with 7.3 x 10
6
m
3
of waste rock, including 5.2 x 10
6
m
3
of potentially acid-generating material. Waste
rock with the highest AP values was placed in the deepest portions of the pit. The waste
was emplaced to elevations within 40 m of the ultimate pit lake surface, and was capped
by a 2 m layer of oxidized, clay-rich material that had no acid-generating potential and
low hydraulic conductivity. As well, large volumes of alluvial material were end-
dumped over the mine walls to lower the slope of the walls and to form a buffer of non-
reactive material between the bedrock and the pit lake and overlying weathering zone.
The lake was then rapidly filled with groundwater pumped at a rate of 560 to 740 L/s
from the same alluvial aquifer that was a burden during the period of active operation. In
14 months, the pit lake surface rose to an elevation that covered all of the reactive waste
and pyrite-bearing bedrock exposed on the mine walls. By contrast, hydrogeological
modeling predicted that – left to its own devices – the lake would have taken over 100
years to fill.
A fortuitous circumstance for the Sleeper pit lake was that the groundwater used to
rapidly fill the pit was not only unusually abundant, but also had good overall quality,
with relatively low TDS, alkaline pH, substantial bicarbonate alkalinity, and very low
toxic metal concentrations. Nonetheless, geochemical modeling prior to flooding
predicted that locally-acidic conditions would occur due to leaching of low pH pore water
from the mine walls and back-filled mine waste. Monitoring of water chemistry during
the initial stages of mine flooding showed that this was indeed the case. It was also
realized that slope failures during or after flooding could cause episodic influxes of acidic
pore water. To mitigate the short- and long-term potential for AMD, the mine pro-
actively added lime during, and for approximately one year after, the period of active
flooding, at a maximum rate of 78 tons/day. An excess of lime was added, with the
anticipation that a turnover event or major landslide after flooding might transport
dissolved metal and acidity accumulated near the bottom of the lake into the upper
portions of the water column. Also, to enhance biological activity in the lake,
approximately 200 tons of manure were added in 4 separate events during and after the
rapid filling program.
In the first two years after the end of the rapid filling program the Sleeper pit lake
underwent several natural turnover events that completely homogenized the water
column. During summer stratification, the epilimnion had 6-9 mg/L of DO, whereas
deeper portions of the lake had little or no DO. After Spring and Fall turnover events, the
DO concentration of the lake was homogenized to values near 5 mg/L. After mixing,
76
concentrations of iron, arsenic, and other contaminants of concern in the lake were lower
than originally predicted by geochemical modeling. This was attributed to precipitation
of copious quantities of HFO when the deep, anoxic water mixed with shallow,
oxygenated water after each turnover event. The freshly precipitated HFO stripped
dissolved arsenic and heavy metals from the water column, and then settled to the bottom
of the lake to accumulate in the benthic sediment.
Monitoring conducted in 2002, roughly 4 years after flooding, showed that the Sleeper pit
lake met all of the State of Nevada water quality standards, with the exception of TDS,
sulfate, and possibly manganese. Although elevated, levels of TDS and sulfate in the
lake after chemical stabilization were actually lower than initially predicted. It remains
to be seen whether or not this lake will become a viable ecosystem for aquatic life. No
information of this type was reported in the paper of Dowling et al. (2004). Because the
lake is terminal, the concentrations of sulfate and TDS are likely to increase with time
owing to evapo-concentration.
5.6. ANCHOR HILL, SOUTH DAKOTA
Between 2001 and 2006, the US EPA and associated technical experts conducted a field-
scale demonstration project to remediate the acidic Anchor Hill pit lake, South Dakota.
This lake (Fig. 2) occupies one of three relatively small open pits at the Gilt Edge Mine
Superfund Site. The mine produced gold between 1986 and 1998, shortly after which
time the parent company declared bankruptcy and the EPA took over administration of
the site. In March 2001, prior to remediation activities, the Anchor Hill lake was 30 m
deep and contained 265,000 m
3
of acidic (pH 3.0) water with highly elevated
concentrations of Al (223 mg/L), Cd (0.6 mg/L), Cu (43 mg/L), Fe (16 mg/L), Mn (27
mg/L), Zn (14 mg/L), sulfate (3270 mg/L), and nitrate (83 mg/L as N) (data from Table 1
of Lewis et al., 2003).
The original goals of the remediation project were twofold (Lewis et al., 2003;
Harrington et al., 2004): 1) to adjust the pH of the entire lake to 7.0±0.2 via lime
addition; and 2) to add organic carbon to stimulate microbial reactions that would
improve water quality to site-specific standards. For the first objective, approximately
265 tonnes of lime were added to the lake in March to May of 2001 using a patented
“Neutra-Mill” system (Earth Systems Pty Ltd, Australia). The Neutra-Mill is essentially
a semi-autonomous floating barge that can dispense lime as it moves across the lake
surface, thereby achieving a better mixing of lime within the lake. Using this method, it
was hoped that a lime efficiency of 85% could be attained. Due in part to practical
difficulties caused by cold winter conditions, the actual lime efficiency that was achieved
was closer to 71% (Lewis et al., 2003), meaning that roughly ¼ of the lime added to the
lake did not initially react with the bulk of the overlying water column. Furthermore, the
pH of the lake drifted down over a period of weeks from values near 7.0 immediately
after lime addition to steady-state values of 4.5 to 5.0. The reason for the drop in pH after
lime addition was not clear, although influx of low pH water during rain events may have
been a contributing factor (Lewis et al., 2003), along with a longer-than expected time for
the lake to reach equilibrium.

77
Following the pH manipulation described above, a series of organic carbon amendments
commenced in May of 2001. The exact sequence of events was complicated, and is
chronicled in detail by Lewis et al. (2003) and Park et al. (2006a, b). In brief, copious
quantities of methanol and animal feed-grade molasses (sometimes with phosphate
added) were pumped into the pit on numerous occasions by use of a hose (Fig. 27). It
was anticipated that the organic carbon amendments would stimulate microbial reactions,
such as reduction of nitrate and sulfate, and that this would generate alkalinity and raise
the pH of the lake. However, the target pH of 7.0±0.2 proved difficult to achieve, and
was not attained until a batch of NaOH solution was added to the lake in September of
2002. Lewis et al. (2003) speculated that high concentrations of dissolved Al may have
inhibited microbial activity at lower pH.
Figure 27. Addition of methanol to the Anchor Hill pit lake. (Photo by Brian Park, May,
2001).
By the summer of 2003, denitrification was essentially complete, and bacterial sulfate
reduction was established, as evidenced by detectable concentrations of H
2
S (Park et al.,
2006a). Dissolved concentrations of most of the metals of concern had dropped to levels
below regulatory standards, although total (unfiltered) concentrations remained
somewhat elevated, presumably due to suspension of fine-grained metal sulfide particles.
Although the water quality of the lake in 2003 was a definite improvement compared to
2001, the presence of excess quantities of H
2
S became a problem, especially in 2004
when levels as high as 100 mg/L were measured in the deeper waters (Park et al., 2006b).
Besides the fact that H
2
S is toxic to most forms of aquatic life, there was a concern for
human health in the event that the lake turned over and released H
2
S gas to the air. As
78
well, the surface of the lake took on a turbid and milky white appearance, due to the
presence of suspended particles of elemental S formed by oxidation of H
2
S. To rectify
the problem of excess H
2
S, yet another large-scale amendment was undertaken in August
2005, this time using 50% hydrogen peroxide (H
2
O
2
) to oxidize H
2
S to S and/or sulfate.
By late 2005, after a probable lake overturn event, most – but not all – of the H
2
S in the
lake had been oxidized. The demonstration project at Anchor Hill effectively ended in
2006, when many millions of gallons of acidic water were transferred from the nearby
Sunday Pit into the Anchor Hill Pit.
Overall, the Anchor Hill project was a success in terms of lowering dissolved metal
concentrations. An important lesson learned was that it can take a very long time (years)
for a pit lake to mix and achieve overall equilibrium after an in situ manipulation in
chemistry. Although preliminary modeling suggested that neutral pH could easily be
attained by a combination of lime addition and microbial reactions, this proved not to be
the case without additional NaOH amendments. Also, the Anchor Hill example shows
that one should not add too large an excess of organic carbon to stimulate microbial
activity, as this may result in undesirably high BOD and H
2
S concentrations, with
associated depletion in dissolved oxygen.
6. ACKNOWLEDGEMENTS
The authors would like to thank Derrick Moggy, Ernie Watson, Julie Dahl, and Marc
Lange of the DFO – Fish Habitat Management Program for support of this project; Jim
Jonas and Karen Smokorowski for helpful peer review comments; and Donna Laroque,
Donna Patterson, and Marge Dyck for administrative support and editing assistance.
Financial and in-kind support for this project was provided by Fisheries and Oceans
Canada and the Montana Department of Environmental Quality.

79
7. REFERENCES CITED
Adams D (1995) Did toxic stew cook the goose? High Country News, Dec. 11, 1995,
http://www.hcn.org/servlets/hcn.Article?article_id=1520
Alberta Environment (2004) Guidelines for lake development at coal mine operations in
the mountains and foothills of the northern East Slopes. 91pp.
Alexeev SV, Alexeeva LP (2003) Hydrogeochemistry of the permafrost zone in the
central part of the Yakutian diamond-bearing province, Russia. Hydrogeology J. 11,
574-581.
Alpers CN, Blowes DW, Nordstrom DK, Jambor JL (1994) Secondary minerals and acid
mine-water chemistry. In Jambor JL, Blowes DW (eds.) Environmental
Geochemistry of Sulfide Mine Wastes. Mineralogical Assoc. Canada, Short Course
22, 247-270.
Alpers CN, Nordstrom DK (1999) Geochemical modeling of water-rock interactions in
mining environments. In Plumlee GS, Logsdon MJ (eds.) The Environmental
Geochemistry of Mineral Deposits. Soc. of Economic Geologists, Reviews in
Economic Geology 6A, 289-324.
Alvarez-Cobelas M, Rubio A, Velasco JL (1990) Chemical limnology of a hypertrophic
gravel-pit lake. Annales de Limnologie ANLIB3, 26, 97-108.
Anderson M, Hawkes CL (1985) Water chemistry of Northern Great Plains strip mine
and livestock watering impoundments. Water Resources Bull., 21, 499-505.
Arauzo M, Cobelas MA, Vicioso J, Verdugo M (1996) The phytoplankton of some
gravel-pit lakes in Spain. Hydrobiologia. 333
, 19-27.
Arkay K (1992) Geothermal energy from abandoned mines: A methodology for an
inventory, and inventory data for abandoned mines in Québec and Nova Scotia.
Geological Survey of Canada, Open File Report 3825, 45 pp.
Armengol X, Miracle MR (2000) Diel vertical movements of zooplankton in lake La
Cruz (Cuenca, Spain). J. Plankton Res. 22, 1683-1703.
ASTM (2007) ASTM D5744-07 Standard Test Method for laboratory weathering of solid
materials using a humidity cell. www.astm.org
.
Axler R, Larsen C, Tikkanen C, McDonald M, Yokom S, Aas P (1996) Water quality
issues associated with aquaculture: a case study in mine pit lakes. Wat Environ Res
68: 995-1011.
Axler R, Yokom S, Tikkanen C, McDonald M, Runke H, Wilcox D, Cady B (1998)
Restoration of a mine pit lake from aquacultural nutrient enrichment. Restor Ecol 6,
1-19.
Baker MJ, Blowes DW, Logsdon MJ, Jambor JL (2003) Environmental geochemistry of
kimberlite materials: Diavik Diamonds Project, Lac de Gras, Northwest Territories,
Canada. Explor. Mining Geol. 10, 155-163.
Baker JP, Schofield CL (1982) Aluminum toxicity to fish in acidic waters. Water Air Soil
Poll. 18, 289-309.
80
Barnes I, O’Neil JR (1969) The relationship between fluids in some fresh alpine-type
ultramafics and possible modern serpentinization, western United States. Geol. Soc.
Am. Bull. 80, 1947-1960.
BHP (1997) NWT diamonds reclamation research studies progress report. BHP
Diamonds, Inc., Yellowknife, NWT, Canada.
BHP (1999) Conceptual strategies for no net loss of fish habitat at the Ekati Mine.
Prepared for BHP Diamonds, Inc., by Rescan Environmental Services, Yellowknife,
NWT, Canada.
BHP (2005) Pit Lake Studies Task 1: Review of the state of knowledge of pit lakes.
Prepared for BHP Billiton Diamonds, Inc., by Rescan Environmental Services LTD,
Yellowknife, NWT, Canada.
Bigham JM, Nordstrom DK (2000) Iron and aluminum hydroxysulfates from acid sulfate
waters. Mineral. Soc. Am., Reviews in Mineralogy and Geochemistry 40, 351-403.
Birtwell IK, Samis SC, Khan NY (2005) Commentary on the management of fish habitat
in northern Canada: information requirements and policy considerations regarding
diamond, oil sands and placer mining – summary reports. Can. Tech. Rep. Fish.
Aquat. Sci. 2607: xii + 65 p.
Blokpoel H (1995) Use of reefraft to create habitat for birds and fish. P. 51-54. In J.R.M.
Kelso and J.H. Hartig [editors]. Methods of modifying habitat to benefit the Great
Lakes ecosystem. CISTI (Can. Inst. Sci. Tech. Inf.) Occas. Pap. No. 1.
Blodau C, Hoffman S, Peine A, Peiffer S (1998) Iron and sulfate reduction in the
sediments of acidic mine lake 116 (Brandenburg, Germany): Rates and geochemical
evaluation. Water Air Soil Poll. 108, 249-270.
Boehrer B, Schultze M (2006) On the relevance of meromixis in mine pit lakes. In:
Barnhisel RI (Ed.), 7
th
Intern. Conf. Acid Rock Drainage (ICARD) Proc., Am. Soc.
Min. Recl. (ASMR), Lexington, Kentucky, pp. 200-213.
Borisov VN, Alexeev SV, Pleshevenkova VA (1995) The diamond mining quarries of
east Siberia as a factor affecting surficial water quality. In: Kharaka YK and Chudaev
OV (Eds.) Proc. 1995 Internatl. Conf. on Water-Rock Interaction, Vladivostok,
Russia, Balkema, pp. 863–865.
Bowell RJ, Parshley JV (2005) Control of pit lake water chemistry by secondary
minerals, Summer Camp Pit, Nevada. Chem. Geol. 215, 373-385.
Brenner FJ, Edmundson J, Werner M, McGrath T (1987) Plankton, chlorophyll
characteristics and fishery potential of surface coal mine lakes in western
Pennsylvania. Proc. Pennsyl. Acad. Sci. 61, 147-152.
Buttleman CG (1992) A handbook for reclaiming sand and gravel pits in Minnesota.
Minn. Dept. Natural Resources, Division of Minerals, 106 pp.
Campbell P, Torgensen T (1980) Maintenance of iron meromixus by iron redeposition in
a rapidly flushed monimolimnion. Can. J. Fish. Aquat. Sci. 37, 1303-1313.
Castendyk DN, Webster-Brown JG (2006) Geochemical prediction and remediation
options for the proposed Martha Mine Pit Lake, New Zealand In: Barnhisel RI (Ed.),

81
7
th
Intern. Conf. Acid Rock Drainage (ICARD) Proc., Am. Soc. Min. Recl. (ASMR),
Lexington, Kentucky, pp. 306–324.
Castro JM, Wielinga BW, Gannon JE, Moore JN (1999) Stimulation of sulfate-reducing
bacteria in lake water from a former open-pit mine through addition of organic
wastes. Water Environ. Res. 71, 218-223.
Castro JM, Moore JN (2000) Pit lakes: their characteristics and the potential for their
remediation. Environ. Geol. 39, 1254-1260.
Cederholm CJ, Bilby RE, Bisson PA, Bumstead TW, Fransen BR, Scarlett WJ Ward JW
(1997) Response of juvenile coho salmon and steelhead to placement of large woody
debris in a coastal Washington Stream. N. Am. J. Fish. Manag. 17, 947–963.
Chapman JT, Coedy W, Schultz S, Rykaart M (2007) Water treatment and management
during the closure of the Colomac Mine. Proc. Mine Closure 2007 Conference,
Santiago, Chile, Oct. 16-19, 2007.
Close TL, Yule D, Siensennop GD (2006) Hydroacoustic methods to estimate stream
trout abundance in Minnesota lakes. Minnesota Dept. of Natural Resources,
Investigational Report 534, 14 pp.
Collienne RJ (1983) Photoreduction in the epilimnion of acidic lakes. Limnol. Oceanogr.
28, 83-100.
Copp GH (1997) Microhabitat use of fish larvae and 0+ juveniles in a highly regulated
section of the River Great Ouse. Regulated River: Research and Management 13(3),
267-276.
Cott PA (2004) Northern pike (Esox lucius) habitat enhancement in the Northwest
Territories. Can. Tech. Rep. Fish. Aquat. Sci. 2528: vii + 32 p.
Cott PA, Hanna BW, Dahl JA (2003) Discussion on seismic exploration in the Northwest
Territories 2000–2003. Can. MS. Rep. Fish. Aquat. Sci. 2648: vi +36.
Cott PA, Sibley P, Somers WM, Lilly MR, Gordon, A (2008a) A review of water level
fluctuations on aquatic biota with an emphasis on fishes in ice-covered lakes. J.
Amer. Water Res. Assoc. 44: 1-17.
Cott PA, Sibley P, Gordon A, Bodaly RA, Mills K, Somers M, Fillatre G (2008b) The
effects of water withdrawal from ice-covered lakes on oxygen, temperature and fish.
J. Amer. Water Resource Assoc., in press.
CWR (2007) Center for Water Research, University of Western Australia.
http://www.cwr.uwa.edu.au/services/models.php?mdid=2
Davis A and Eary LE (1997) Pit lake water quality in the western United States: An
analysis of chemogenetic trends. Min. Eng., Jun, 98-102.
Davis A (2003) A screening-level laboratory method to estimate pit lake chemistry.
Mine Water Environ. 22, 194-205.
Davison W, George DG, Edwards NJA (1995) Controlled reversal of lake acidification
by treatment with phosphate fertilizer. Nature 377, 504-507.

82
Day S, Sexsmith K., Millard J. (2003) Acidic drainage from calcareous coarse kimberlite
reject. EKATI diamond mine, NWT, Canada. 6
th
Internatl Conf. Acid Rock
Drainage, Cairns, Australia.
Dessouki TCE, Hudson JJ, Neal BR, Bogard MJ (2005) The effects of phosphorous
additions on the sedimentation of contaminants in a uranium mine pit-lake. Water
Research 39, 3055-3063.
DFO (1986) The Department of Fisheries and Oceans, Policy for the Management of Fish
Habitat. Department of Fisheries and Oceans, Ottawa, Ont.
Diez Ercilla M, López Pamo E., Sánchez España J. (in press) Photoreduction of Fe(III) in
the acidic mine pit lake of San Telmo (Iberian Pyrite Belt): Field and experimental
work. Aquat. Geochem., in press.
Diavik Diamond Mine Incorporated (2007) Diavik Fact Book: The Diavik Diamond
Mine. http://www.diavik.ca/PDF/DiavikFactBook.pdf
. Accessed on 2007-012-15.
Di Toro DM, Allen HE, Bergman HL, Meyer JS, Paquin PR, Santore RC (2001) A biotic
ligand model of the acute toxicity of metals I. Technical basis. Environ. Toxicol.
Chem. 20, 2383–2396.
Donnelly CL, Stachel T, Creighton S, Muehlenbachs K, Whiteford S (2007) Diamonds
and their mineral inclusions from the A154 South pipe, Diavik Diamond Mine,
Northwest Territories, Canada. Lithos 98, 160-176.
Doran PT, McKay CP, Adams WP, English MC, Wharton RA, Meyer MA (1996)
Climate forcing and thermal feedback of residual lake-ice covers in the high Arctic.
Limnol. Oceanogr. 41, 839-848.
Doudoroff D, Shumway DL (1970) Dissolved oxygen requirements of freshwater fishes.
Food and Agricultural Organization of the United Nations. FAO Fisheries Technical
Paper No. 86.
Doupé RG, Lymbery AJ (2005) Environmental risks associated with beneficial end uses
of mine lakes in Southwestern Australia. Mine Water Environ. 24, 134-138.
Dowling J, Atkin S, Belae G, Alexander G (2004) Development of the Sleeper Pit Lake.
Mine Water Environ. 23, 2-11.
Doyle GA, Runnells DD (1997) Physical limnology of existing mine pit lakes. Min.
Eng., Dec. 76-80.
Drever JI (1997) The Geochemistry of Natural Waters, 3
rd
ed. Prentice-Hall.
Driscoll CT Jr, Baker JP, Bisogni JJ Jr, Schofield CL (1980) Effect of aluminium
speciation on fish in dilute acidified waters. Nature 284, 161-164.
Duguay CR, Flato GM, Jeffries MO, Menard P, Morris K, Rouse WR (2003) Ice-cover
variability on shallow lakes at high latitudes: model simulations and observations.
Hydrological Processes 17, 3465-3483.
Eary LE (1999) Geochemical and equilibrium trends in mine pit lakes. Appl. Geochem.
14, 963-987.
Eary LE (1998) Predicting the effects of evapoconcentration on water quality in mine pit
lakes. J. Geochem. Expl. 64, 223-236.

83
Emmenegger L, Schönenberger R, Sigg L, Sulzberger B (2001) Light-induced redox
cycling of iron in circumneutral lakes. Limnol. Oceanogr. 46, 49-61.
Environment Canada (2004) Threats to water availability in Canada. National Water
Research Institute, Burlington, Ontario. NWRI Scientific Assessment Report Series
No. 3 and ACSD Science Assessment Series No. 1. 128 pp.
Evans DO (2005) Effects of hypoxia on scope-for-activity of lake trout: defining a new
dissolved oxygen criterion for protection of lake trout habitat. Technical Report 2005-
01. Habitat and Fisheries Unit, Aquatic Research and Development Section, ARDB,
Peterborough, ON. 19 pp.
Evans CE, Reist JD, Minns CK (2002) Life history characteristics of freshwater fishes
occurring in the Northwest Territories and Nunavut, with major emphasis on riverine
habitat requirements. Can. MS Rep. Fish. Aquat. Sci. 2614, xiii + 169 pp.
Fauville AB, Mayer R, Frömmichen R, Friese K, Veizer J (2004) Chemical and isotopic
evidence for accelerated bacterial sulphate reduction in acid mining lakes after
addition of organic carbon: laboratory batch experiments. Chem. Geol. 204, 325-344.
FII (2008) Floating Island International, Biohaven Floating Islands.
http://www.floatingislandinternational.com/index.phtml
, accessed May, 2008.
Fisher TSR, Lawrence G (2006) Treatment of acid rock drainage in a meromictic mine
pit lake. J. Environ. Eng. 132, 515-526.
Fitzsimons JD (1996) The significance of man-made structures for lake trout spawning in
the Great Lakes: are they a viable alternative to natural reefs? Can. J. Fish. Aquat.
Sci. 53(Suppl. 1), 142-151.
Foster NR, Kennedy GW (1995) Patterns of egg deposition by lake trout and
lake
whitefish at Tawas artificial reef, Lake Huron, 1990-l 993, p. 191-206. In M.
Munawar, T. Edsall, and J. Leach [ed.] The Lake Huron ecosystem: ecology, fisheries
and management. Ecovision World Mono. Series, SPB Academic
Publishing, the
Netherlands.
Friese K, Herzsprung P, Witter B (2002) Photochemical degradation of organic carbon in
acidic mining lakes. Acta Hydrochim. Hydrobiol. 30, 141-148.
Friese K, Hupfer M, Schultze M (1998) Chemical characteristics of water and sediment in
acid mining lakes of the lusatian lignite district. In: Geller W, Klapper H, Solomons W
(Eds.), Acidic Mining Lakes: Acid Mine Drainage, Limnology, and Reclamation, pp. 25-
46.
Frömmichen R, Kellner S, Friese K (2003) Sediment conditioning with organic and/or
inorganic carbon sources as a first step in alkalinity generation of acid mine pit lake
water (pH 2-3). Environ. Sci. Technol. 37, 1414-1421.
Gaboury MN, Patalas JW (1984) Influence of water level drawdown on the fish
populations of Cross Lake, Manitoba. Can. J. Fisheries Aquat. Sci. 41, 118-125.
Gammons CH, Duaime TE (2006) Long term changes in the limnology and geochemistry
of the Berkeley Pit Lake Butte, Montana. Mine Water Environ. 25, 76-85.
Gammons CH, Metesh JJ, Snyder DM (2006a) A survey of the geochemistry of flooded
mine shaft water in the Butte District, Montana. Mine Water Environ. 25, 100-107.

84
Gammons CH, Nimick DA, Parker SR, Cleasby TE, McCleskey RB (2005) Diel behavior
of Fe and other heavy metals in a mountain stream with acidic to neutral pH: Fisher
Creek, Montana, USA. Geochim. Cosmochim. Acta 69, 2505-2516.
Gammons CH, Nimick DA, Parker SR, Snyder DM, McCleskey RB, Amils R, Poulson
SR (2008) Photoreduction fuels biogeochemical cycling of iron in Spain’s acid rivers.
Chem. Geol., in press, doi:10.1016/j.chemgeo.2008.03.004
.
Gammons CH, Poulson SR, Pellicori DA, Roesler A, Reed PJ, Petrescu EM (2006b) The
hydrogen and oxygen isotopic composition of precipitation, evaporated mine water,
and river water in Montana, USA. J. Hydrology 328, 319-330.
Geller W, Klapper H, Solomons W (1998) Natural and anthropogenic sulfuric acidification of
lakes. In: Geller W, Klapper H, Solomons W (Eds.), Acidic Mining Lakes: Acid Mine
Drainage, Limnology, and Reclamation, Springer, New York, pp. 3-24.
Ghomshei MM (2007) Geothermal energy from Con Mine for heating the city of
Yellowknife, NWT: A concept study.
http://www.yellowknife.ca/__shared/assets/Geothermal_Study_Final_Report6259.pdf
Gibb JP, Evans RL (1978) Preliminary evaluation of final cut lakes. Illinois State Water
Survey, Circular 130, 89pp.
Gibson JAE (1999a) The role of ice in determining mixing intensity in Ellis Fjord,
Vestford Hills, Antarctica. Antarctic Science 11, 419-426.
Gibson JAE (1999b) The meromictic lakes and stratified marine basins of the Vestfold
Hills, East Antarctica. Antarctic Science 11, 175-192.
Grillmayer RG (1995) Soil Bioengineering Technical Report: Black Ash Creek
Rehabilitation Project, 1992-1994. Collingwood Harbour Remedial Action Plan.
Report #ISBN 0-7778-4080-4, Ontario Ministry of the Environment, Toronto,
Ontario, Canada.
Godwin AL, Conly AG, Lee PF (2007) Predictive modeling of water quality issues of
two pit lakes at the former Steep Rock iron mine, northwestern Ontario. Geol. Soc.
Amer., Abstr. Prog. 39(6), p. 101.
Gray JE, Greaves IA, Bustos DM, Krabbenhoft DP (2003) Mercury and methylmercury
contents in mine-waste calcine, water, and sediment collected from the Palawan
Quicksilver Mine, Phillipines. Environ. Geol. 43, 298-307.
Greenbank JT (1945) Limnological conditions in ice-covered lakes, especially as related
to winter-kill of fish. Ecol. Monogr. 15, 343-392.
Guilbert JM, Park CF Jr. (1986) The Geology of Ore Deposits, New York, W.H.
Freeman.
Hanna TM, Azrag EA, Atkinson LC (1994) Use of an analytical solution for preliminary
estimates of groundwater inflow to a pit. Mining Eng., Feb. 1994, 149-152.
Harrington JG, Wangerud K, Fundingsland SD (2004) Restoration of ecosystems and
long-term stabilization of initially acidic pit lakes, Gilt Edge Mine Superfund Site,
South Dakota. Proc. Tailings and Mine Waste ’04, Taylor & Francis Group, London,
pp. 399-406.
85
Hatch J T, Schmidt KP, Siems DP, Underhill JC, Bellig RA, Baker RA (2003) A new
distributional checklist of Minnesota fishes, with comments on historical significance.
J. Minn. Acad. Sci. 67, 1–17.
Headley TR, Tanner CC (2006) Application of floating wetlands for stormwater
treatment: A review. National Inst. Water Atmospheric Res., Aukland, New Zealand,
NIWA Client Report HAM2006-123, 100pp.
Heginbottom JA, Dubreuil MA, Harker PA (1995) Canada - Permafrost, in: National
Atlas of Canada, 5th Edition, National Atlas Information Service, Natural Resources
Canada, MCR 4177.
Helmer C, Labroue L (1993) Denitrification in gravel-pit lakes. Hydrobiologia 252, 35-
44.
Hodges BR, Imberger J, Saggio A, Winters KB (2000) Modeling basin-scale internal
waves in a stratified lake. Limnol. Oceanogr. 45, 1603-1620.
Hongve D (1997) Cycling of iron, manganese, and phosphate in a meromictic lake.
Limnol. Oceanogr. 42, 635-647.
Holowenko FM, MacKinnon MD, Fedorak PM (2000) Methanogens and sulfate-reducing
bacteria in oil sands fine tailings waste. Can. J. Microbiol. 46, 927-937.
Hutchinson GE (1957) A Treatise on Limnology. I. Geography, Physics, and Chemistry.
John Wiley & Sons, New York.
Imberger J, Patterson JC (1981) A dynamic reservoir simulation model - DYRESM:5. In:
H. Fischer (Ed.), Transport Models for Inland and Coastal Waters, Academic Press,
New York, pp. 310-361.
INAC (2007) Indian and Northern Affairs Canada, Mine Site Reclamation Guidelines for
the NWT. Yellowknife, NT. v + 51p.
Jonas JP (2000) Current seasonal limnology of the Berkeley Pit Lake, In: 5
th
Intern. Conf.
Acid Rock Drainage (ICARD) Proc., Soc. Min. Metall. Expl. (SME), Littleton,
Colorado, pp. 359-366.
Jones NE, Tonn WM, Scrimgeour GJ, Katapodis C (2003) Productive capacity of an
artificial stream in the Canadian Arctic: assessing the effectiveness of fish habitat
compensation. Can. J. Fish. Aquat. Sci. 60, 849-869.
Kalin M, Cao Y, Smith M, Olaveson MM (2001) Development of the phytoplankton
community in a pit-lake in relation to water quality changes. Water Res. 35, 3215-
3225.
Kelch DO, Reutter J (1995) Artificial reefs in Lake Erie: A habitat enhancement tool. In
Kelso JRM, Hartig JH (Eds.) Methods of modifying habitat to benefit the Great Lakes
ecosystem. CISTI (Can. Inst. Sci. Tech. Inf.) Occas. Pap. No. 1, pp.243-249.
Kelly MG (1988) Mining and the Freshwater Environment. Elsevier, London, 231pp.
Kelly MG (1999) Effects of heavy metals on the aquatic biota. In Plumlee GS, Logsdon
MJ (eds.) The Environmental Geochemistry of Mineral Deposits. Soc. of Economic
Geologists, Reviews in Economic Geology 6A, 363-371.

86
Klapper H, Friese K, Scharf B, Schimmele M, Schultze M (1998) Ways of controlling
acid by ecotechnology. In: Geller W, Klapper H, Salomons W (Eds.), Acidic Mining
Lakes. Springer-Verlag, Berlin, pp. 401–418.
Klapper H, Geller W (2001) Water quality management of mining lakes – a new field of
applied hydrobiology. Acta Hydrochim. Hydrobiol. 29, 363-374.
Klapper H, Schultze M (1995) Geogenically acidified mining lakes – living conditions
and possibilities of restoration. Int. Rev. Ges. Hydrobiol. 80, 639–653.
Klein C, Hurlbut CS Jr.(1993) Manual of Mineralogy, Twenty-First Edition, John Wiley
and Sons.
Konrad JM, McGammon AW (1990) Solute partitioning in freezing soils. Canad.
Geotechnical J. 67, 726-736.
Koschorrek, M, Frommchen R, Herzsprungand P, Wendt-Potthoff K (2002) Function of
straw for in situ remediation of acidic mining lakes. Water Air Soil Pollut. 2, 97–109.
Koski KV (1992) Restoring stream habitats affected by logging activities. In Thayer GW
(Ed.) Restoring the nation's marine environment. A Maryland Sea Grant Book,
College Park, Md., pp. 344-397.
KTH (2007) Visual MINTEQ ver 2.53. KTH, Department of Land and Water Resources
Engineering. http://www.lwr.kth.se/English/OurSoftware/vminteq/
Kuchling K, Eng P, Chorley D, Geo P, Zawadzki W (2000) Hydrogeological modeling of
mining operations at the Diavik Diamonds project. In Singhai RK, Mehrotra AK
(Eds.) Environmental Issues and Management of Waste in Energy and Mineral
Production. Taylor and Francis.
Lacelle D, Doucet A, Clark ID, Lauriol B (2007) Acid drainage generation and seasonal
recycling in disturbed permafrost near Eagle Plains, northern Yukon Territory,
Canada. Chem. Geol. 243, 157-177.
Langmuir D (1997) Aqueous Environmental Geochemistry. Prentice-Hall.
Lapakko KA (2003) Developments in humidity-cell tests and their application. In
Jambor JL, Blowes DW, Ritchie AIM (Eds.) Environmental Aspects of Mine Wastes.
Mineralogical Assoc. Canada, Short Course 31, 147-164.
Ledin M, Pederson K (1996) The environmental impact of mine wastes; roles of
microorganisms and their significance in treatment of mine wastes. Earth Sci. Rev.
41, 67-108.
Lessmann D, Nixdorf B (2000) Acidification control of phytoplankton diversity, spatial
distribution and trophy in mining lakes. Verh. Internat. Verein. Limnol. 27, 2208–
2211.
Lessmann D, UhlmannW, Gunewald U, Nixdorf B (2003) Sustainability of the flooding
of lignite mining lakes as a remediation technique against acidification in the Lusatian
mining district, Germany. Proc. 6
th
Internat. Conf. on Acid Rock Drainage, Cairns,
Qld, 521–527.
87
Levy DB, Custis KH, Casey WH, Rock PA (1997) The aqueous geochemistry of the
abandoned Spenceville copper pit, Nevada County, California. J. Environ. Qual. 26,
20-48.
Lewis NM, Wangerud KW, Park BT, Fundingsland SD, Jonas JP (2003) Status of in situ
treatment of Anchor Hill Pit Lake, Gilt Edge Mine Superfund Site, South Dakota,
USA. In: Farrell T, Taylor G, (Eds.) 6
th
Intern. Conf. Acid Rock Drainage (ICARD),
Australasian Institute of Mining and Metallurgy (AusIMM), Carlton, Australia, pp.
779-788.
Lipsey LL, Malcolm S (1981) Summer zooplankton communities of selected borrow-pit
ponds in northern Illinois. Hydrobiologia 77, 81-85.
Livingstone DA (1963) Alaska, Yukon, Northwest Territories, and Greenland. In Frey
DG (Ed.) Limnology of North America, Univ. of Wisconsin Press, pp. 559-574.
Lovley DR and Phillips EJP (1992) Bioremediation of uranium contamination with
enzymatic uranium reduction. Environ. Sci. Technol. 26, 2226-2234.
Luscar Ltd (1993) Development of sport fisheries in lakes created by coal mining
operations in the Eastern Slopes. Progress Report #5: Results of limnological
investigations of study lakes conducted in July 1993. 56 pp.
Mackay JR (1997) A full-scale field experiment (1978-1995) on the growth of permafrost
by means of of lake drainage, western Arctic coast: a discussion of the method and
some results. Canadian J Earth Sciences 34, 17-33.
Mackay JR Burn CR (2002) The first 20 years (1978-1979 to 1998-1999) of ice-wedge
growth at the Illisarvik experimental drained lake site, western Arctic coast, Canada.
Canadian J Earth Sciences 39, 95-111.
Madison JP, Gammons CH, Poulson SR, Jonas JP (2003) Oxidation of pyrite by ferric
iron in the acidic Berkeley Pit Lake,Montana, USA. In: Farrell T, Taylor G, (Eds.) 6th
Intern. Conf. Acid Rock Drainage (ICARD) Proc., Australasian Institute of Mining
and Metallurgy (AusIMM), Carlton, Australia, pp. 1073-1078.
Maest AS, Metesh JJ, Duaime TE (2004) Mass balance modeling of dissolved copper
loading to the Berkeley Pit, Montana, USA, and estimates of pit wall leaching. Proc,
11
th
Water-Rock Interaction Symp, Saratoga Springs, NY, Balkema, Taylor &
Francis Group, London, Vol. 2, 1569-1573.
Magnuson JJ, Paszkowski CA, Rahel FJ, Tonn WM (1989) Fish ecology in severe
environments of small isolated lakes in northern Wisconsin. In Sharitz RR, Gibson
JW (Eds.) Freshwater wetlands and wildlife. Office of Scientific and Technical
Information, Department of Energy, Oak Ridge, Tenn. Symp. Ser. No. 61. pp. 487–
515.
Marinelli F, Niccoli W (2000) Simple analytical equations for estimating ground water
inflow to a mine pit. Ground Water 38, 311-314.
McAfee ME (1980) Effects of water drawdown on the fauna in small cold water
reservoirs. Water Res. Bull. 16, 690-696.
McBirney AR (1993) Igneous Petrology, Second Edition, Jones and Bartlett, 435-441.

88
McCullough CD (2007) Approaches to remediation of acid mine drainage water in pit
lakes. Int. J. Mining, Reclamation, and Environment. DOI:
10.1080/17480930701350127.
McCullough CD, Lund MA (2006) Opportunities for sustainable mining pit lakes in
Australia. Mine Water Environ. 25, 220-226.
McKnight DM, Kimball BA, Bencala KE (1988) Iron photoreduction and oxidation in an
acidic mountain stream. Science 240, 637-640.
McNaughton K, Lee PF, Lindsay D, Sudbury MP (1999) The limnology of an open pit
fish farm. Mine Water Environ. 1, 169-175.
MERG (2003) Detoxifying pit lakes by controlled algal blooms: Laboratory test and pilot
phyotremediation trail at Little Creek Pond at Vangorda Pit near Faro, Yukon
Territory, Canada. Prepared for Mining Environment Research Group (MERG) and
Environment Canada, by Microbial Technologies Inc. and Laberge Environmental
Services, 57 pp.
Michelutti N, Wolfe AP, Vinebrooke RD, Rivard B, Briner JP (2005) Recent primary
productivity production increases in arctic lakes. Geophys. Res. Lett. 32, L19715,
doi:10.1029/2005GL023693.
Miller GC, Lyons WB, Davis A (1996) Understanding the water quality of pit lakes.
Environ. Sci. Technol. 30, 118A-123A.
Mills C (2007) Acid Base Accounting (ABA) test procedures.
http://technology.infomine.com/enviromine/ard/Acid-
Base%20Accounting/acidbase.htm, accessed 12/07.
Mills KH, Chalanchuk SM, Allan DJ, Bodaly RA (2002) Abundance, survival, condition,
and recruitment of lake whitefish (Coregonus clupeaformis) in a lake subjected to
winter drawdown. Arch. Hydrobiol. 57, 209-219.
Minewater Project (2007) http://www.minewaterproject.info/
, accessed December 2007.
Morin KA, Hutt NM (2001) Prediction of water chemistry in mine lakes: The minewall
technique. Ecological Eng. 17, 125-132.
Morton KL, Müller S (2003) Hydrogeology of the Venetia diamond mine, South Africa.
S. African J. Geology 106, 193-204.
Moy PB (1995) Burns Harbour major rehabilitation – submerged breakwater reefs. In
Kelso JRM, Hartig JH (Eds.) Methods of modifying habitat to benefit the Great Lakes
ecosystem. CISTI (Can. Inst. Sci. Tech. Inf.) Occas. Pap. No. 1, p.250-254.
Moyle PB, Cech JJ (2003) Fishes: An Introduction to Ichthyology, 5
th
ed. Benjamin
Cummings.
Murphy WM (1997) Are pit lakes susceptible to limnic eruptions? In: Tailings and Mine
Waste ’97, Balkema, Rotterdam, Holland, pp. 543-547.
Naugle GD, Atkinson LC (1993) Estimating the rate of post-mining filling of pit lakes.
Mining Eng. 45, 402-404.

89
Naylor R, Hindar K, Fleming IA, Goldburg R, Williams S, Volpe J, Whoriskey F, Eagle
J, Kelso D, Mangel M (2005) Fugitive salmon: Assessing the risks of escaped fish
from net-pen aquaculture. BioScience 55, 427-437.
Nelson JS, Paetz MJ (1992) The Fishes of Alberta. Univ. of Alberta, 437pp.
NETL (2006) Underground mine water for heating and cooling using geothermal heat
pump systems. National Energy Technol. Lab., U.S. Dept. of Energy,
http://www.netl.doe.gov/publications/factsheets/rd/R&D088.pdf.
Newbrey MG, BozekMA, Jennings M, Cook JE (2005) Branching complexity and
morphological characteristics of coarse woody structure as lacustrine fish habitat.
Can. J. Fish. Aquat. Sci. 62, 2110–2123.
Newbrough P, Gammons CH (2002) Experimental investigation of water-rock
interaction and acid mine drainage at Butte, Montana. Environ. Geol. 41, 705-719.
Nickum JG (1970) Limnology of winterkill lakes on South Dakota. Presented at the
annual meeting held in conjunction with the 32
nd
Midwest Fish and Wildlife
Conference. North Central Division, American Fisheries Society.
Nixdorf B, Lessmann D, Deneke R (2005) Mining lakes in a disturbed landscape:
Application of the EC Water Framework Directive and future management strategies.
Ecol. Eng. 24, 67-73.
Nixdorf B, Mischke U, Lessmann D (1998a) Chrysophytes and chlamydomonads:
pioneer colonists in extremely acidic mining lakes (pH < 3) in Lusatia (Germany).
Hydrobiologia 369/370, 315–327.
Nixdorf B, Wollmann K, Deneke R (1998b) Ecological potential for planktonic
development and food web interactions in extremely acidic mining lakes in Lusatia.
In: Geller W, Klapper H, Salomons W (Eds.), Acidic Mining Lakes. Springer-Verlag,
Berlin, Heidelberg, pp. 147–167.
Niyogi S, Wood CM (2004) The Biotic Ligand Model, a flexible tool for developing site-
specific water quality guidelines for metals. Environ. Sci. Technol. 38, 6177-6192.
Nordstrom DK, Alpers CN (1999) Geochemistry of acid mine waters. Society of
Economic Geologists, Reviews in Economic Geology 6A, 133-160.
Nordstrom DK (2003) Effects of microbial and geochemical interactions in mine
drainage. In J.L. Jambor, D.W. Blowes, A.I.M. Ritchie, eds., Environmental Aspects
of Mine Wastes, Mineral. Assoc. Canada, Short Course 31, 227-238.
Norris DE (2004) Mosquito-borne diseases as a consequence of land use change.
Ecohealth 1, 19-24.
Northcote TG and Larkin PA (1963) Western Canada. In Frey DG (Ed.) Limnology of
North America, Univ. of Wisconsin Press, pp. 451-486.
Nowicki T, Crawford B, Dyck D, Carlson J, McElroy R, Oshust P, Helmstaedt H (2004)
The geology of kimberlite pipes of the Ekati property, Northwest Territories, Canada.
Lithos 76, 1-27.
NRC
Canada (1988) Glossary of permafrost and related ground-ice terms, Natural
Resources Canada, Technical Memorandum 142, Ottawa, Ontario, Canada.
90
Ogawa K, Nakatsugawa T, Yasuzaki IM (2004) Heavy metacercarial infections of
cyprinid fishes in Uji River. Fisheries Science 70, 132–140.
Oremland RS, Hollibaugh JT, Maest AS, Presser TS, Miller LG, Culbertson CW (1989)
Selenate reduction to elemental selenium by anaerobic bacteria in sediments and
culture: Biogeochemical significance of a novel, sulfate-independent respiration.
Appl. Environ. Microbiol. 55, 2333-2343.
Otchere FA, Veiga MM, Hinton JJ, Farias RA, Hamaguchi R (2004) Transforming open
mining pits into fish farms: moving towards sustainability. Nat. Resources Forum 28,
216-223.
Ownbey GB, Morley T (1991) Vascular Plants of Minnesota, A Checklist and Atlas.
Univ. of Minnesota Press, Minneapolis, 307 pp.
Pagenkopf GK (1983) Gill surface interaction model for trace-metal toxicity to fishes:
role of complexation, pH, and water hardness. Environ. Sci. Technol. 17, 342–347.
Paquin P R, Gorsuch JW, Apte S, Batley GE, Bowles KC, Campbell PGC, Delos CG, Di
Toro DM, Dwyer RL, Galvez F, Gensemer RW, Goss GG, Hogstrand C, Janssen CR,
McGeer JC, Naddy RB, Playle RC, Santore RC, Schneider U, Stubblefield WA,
Wood CM, Wu KB (2002) The biotic ligand model: a historical overview. Comp.
Biochem. Physiol. C. 133, 3-35.
Paquin PR, Santore RC, Wu KB, Kavvadas CD, Di Toro DM (2000) The biotic ligand
model: a model of the acute toxicity of metals to aquatic life. Environ. Sci. Policy 3,
S175-S182.
Park BT, Wangerud KW, Fundingsland SD, Adzic ME, Lewis NM (2006a) In situ
chemical and biological treatment leading to successful water discharge from Anchor
Hill pit lake, Gilt Edge Mine superfund site, South Dakota, U.S.A. Proc. 7
th
Internat.
Conf. Acid Rock Drainage, St. Louis, MO, USA, pp. 1065-1069.
Park BT (2006b) Update on two-stage in situ treatment of Anchor Hill pit lake, Gilt Edge
Mine Superfund Site. Proc. 2006 Mine Design, Operations and Closure Conference,
Fairmont Hot Springs, Montana.
Peine A, Peiffer S (1998) In-lake neutralization of acid mine lakes. In Geller W, Klapper
H, Salomons W (Eds.) Acidic Mining Lakes. Springer, pp. 47-63.
Pellicori DA, Gammons CH, Poulson SR (2005) Geochemistry and stable isotope
composition of the Berkeley pit lake and surrounding mine waters, Butte, Montana.
Appl. Geochem. 20, 2116-2137.
Phylips VE (1992) Variation in winter wildfowl numbers on gravel pit lakes at Great
Linford, Buckinghamshire, 1974-79 and 1984-91, with particular reference to the
effects of fish removal. Bird Study 39, 177-185.
Pienitz R, Smol JP, Lean DRS (1997a) Physical and chemical limnology of 24 lakes
located between Yellowknife and Contwoyto Lake, Northwest Territories (Canada).
Canadian J. Fisheries Aquat. Sci. 54, 347-358.
Pienitz R, Smol JP, Lean DRS (1997b) Physical and chemical limnology of 59 lakes
located between the southern Yukon and the Tuktoyaktuk Peninsula, Northwest
Territories (Canada). Canadian J. Fisheries Aquat. Sci. 54, 330-346.
91
Pieters R, Lawrence G (2007) Circulation of Tailings Lake and Zone 2 Pit-Lake,
Colomac NWT, 2006. Prepared for the Indian and Northern Affairs Contaminants
and Remediation Directorate, 17pp + figures and appendices.
Pietsch W (1993) Gewassertypen der Begbaugebiete des Lausitzer Braunkohlereviers
und Makrophytenverbreitung.
In: Abschlullbericht zum Forschungsprojekt
.,Erarbeitung der wissenschaftlichen Grundlagen fur die Gestaltung, Flutung und
Wassergutebewirtschaftung von Bergbaurestseen".BMFT-Forderkennzeichen
Porsild AE, Cody WJ (1980) Vascular Plants of Continental Northwest Territories.
National Museums of Canada, Ottawa. 667pp.
Pratt TC, Smokorowski KE (2003) Fish habitat management implications of the summer
habitat use by littoral fishes in a north temperature, mesotrophic lake. Can. J. Fish
Aquat. Sci. 60, 286-300.
Quigley JT, Harper DJ, Galbraith RV (2006) Fish habitat compensation to achieve no net
loss: Review of past practices and proposed future directions. Can. Tech. Rep. Fish.
Aquat. Sci. 2632: vi + 22p.
Ramstedt M, Carlsson E, Lövgren L (2003) Aqueous geochemistry in the Udden pit lake,
northern Sweden. Appl. Geochem. 18, 97-108.
Randall SR, Sherman DM, Ragnarsdottir KV (2001) Sorption of As(V) on green rust
(Fe
4
(II)Fe
2
(III)(OH)
12
SO
4
·3H
2
O) and lepidocrocite (γ-FeOOH): Surface complexes
from EXAFS spectroscopy. Geochim. Cosmochim. Acta 65, 1015-1023.
Redfield AC, Ketchum BJ, Richards FA (1963) The influence of organisms on the
composition of sea water. In Hill MN (Ed.), The Sea (Vol. 2). Wiley –Interscience,
New York, pp. 26-77.
Reid NB, Naeth MA (2005a) Establishment of a vegetation cover on tundra kimberlite
mine tailings: A greenhouse trial. Restoration Ecology. 13(4), 593-600.
Reid NB, Naeth MA (2005b) Establishment of a vegetation cover on tundra kimberlite
mine tailings: A field trial. Restoration Ecology. 13(4), 601-608.
Rikiishi K, Hashiya E, Imai M (2004) Linear trends of the length of snow-cover season in
the Northern Hemisphere as observed by the satellites in the period 1972-2000
Annals of Glaciology 38, 229-237.
Roch M, Nordin RN, Austin A, Mckean CPG, Deniseger J, Kathman RD, McCarter JA,
Clark MRJ (1985) The effects of heavy metal contamination on the aquatic biota of
Buttle Lake and Campbell river drainage (Canada). Archives Environ. Contam.
Toxicol. 14, 347–362.
Roth BM, Kaplan IC, Johnson PT, Sugden-Newbery A, Sass GG, Havlicek TD, Willis
TV, Turner MG, Carpenter SR (2007) Linking terrestrial and aquatic ecosystems: The
role of woody habitat in lake food webs. Ecological Modeling 203, 439-452.
Rollo HA, Jamieson HE (2006) Interaction of diamond mine waste and surface water in
the Canadian Arctic. Appl. Geochem. 1522-1538.
Roni P, Hanson K, Beechie T, Pess G, Pollock M, Bartley DM (2005) Habitat
rehabilitation for inland fisheries. Global review of effectiveness and guidance for
92
rehabilitation of freshwater ecosystems. FAO Fisheries Technical Paper, No. 484.
Rome, FAO. 116p.
Roulet NT, Adams WP (1984) Illustration of the spatial variability of light entering a lake
using an empirical-nodel. Hydrobiologia 109, 67-74.
Rytuba JJ, Enderlin D, Ashley R, Seal R, Hunerlach MP (2000) Evolution of the
McLaughlin Gold Mine pit lakes, California. Proc. 5
th
International Conf. on Acid
Rock Drainage, pp. 367-375.
Sader J, Leybourne MI, McClenaghan MB, Hamilton SM (2007) Low-temperature
serpentinization processes and kimberlite groundwater signatures in the Kirkland
Lake and Lake Timiskiming kimberlite fields, Ontario Canada: implications for
diamond exploration. Geochemistry: Exploration, Environment, Analysis 7, 3-21.
Sánchez-España J, López-Pamo E, Santorimia E, Diez-Ercilla M (2007) Different types
of hydrochemical stratification in the acidic mine pit lakes of the Iberian Pyrite Belt.
Proc. 17
th
V. M. Goldschmidt Conf., Cologne, Germany, August 2007, Geochim.
Cosmochim. Acta 71-15(Suppl. 1), p. A871.
Santore RC, Di Toro DM, Paquin PR, Allen HE, Meyer JS (2001) Biotic ligand model of
the acute toxicity of metals. 2. Application to acute copper toxicity in freshwater fish
and daphnia. Environ. Toxicol. Chem. 20, 2397-2402.
Santoul F, Figuerola J, Green AJ (2004) Importance of gravel pits for the conservation of
waterbirds in the Garonne river floodplain (southwest France). Biodiversity and
Conservation 13, 1231-1243.
Schmid M, Lorke A, Dinkel C, Tanyileke G, Wuest A (2004) Double-diffusion
convection in Lake Nyos, Cameroon. Deep-Sea Research I 51, 1097-1111.
Schwartz T (2002) Fish populations, biomass, and growth in Lac des Roches, Alberta. A
report by Pisces Environ. Consult. Ltd., Red Deer, AB for Cardinal River Coals Ltd.,
Hinton, AB, 88pp.
Scott WB, Crossman EJ (1998) The Freshwater Fishes of Canada. Oakville, Ontario,
Canada: Galt House Publications Ldt..
Shevenell L (2000a) Water quality in pit lakes in disseminated gold deposits compared to
two natural, terminal lakes in Nevada. Environ. Geol. 39, 807-815.
Shevenell L (2000b) Analytical method for predicting filling rates of mining pit lakes:
Example from the Getchell Mine, Nevada. Min. Eng. 52, 53-60.
Shevenell L, Connors KA, Henry CD (1999) Controls on pit lake water quality at sixteen
open-pit mines in Nevada. Appl. Geochem. 13, 669-687.
Sheals J, Granström M, Sjöberg S, Persson P (2003) Coadsorption of Cu(II) and
glyphosate at the water-goethite (alpha-FeOOH) interface: molecular structures from
FTIR and EXAFS measurements. J. Colloid Interface Sci. 262, 38-47.
Sibley PK, White DM, Cott PA, Lilly MR (2008) Water use from arctic lakes:
identification, impacts, and decision support. J. Amer. Water Res. Assoc. 44, 1-3.
93
Sinclair G, Fawcett M (1994) Development of an aquatic habitat and water resource in
closing out the Enterprise Pit. In: Publications of the Australasian Institute of Mining
and Metallurgy 5/94. Australasian Inst Min Metall, Melbourne, pp 447–457.
Smith KS (1999) Metal sorption on mineral surfaces: An overview with examples
relating to mineral deposits. In Plumlee GS, Logsdon MJ (Eds.) The Environmental
Geochemistry of Mineral Deposits. Soc. of Economic Geologists, Reviews in
Economic Geology 6A, 161-182.
Smokorowski KE, Withers KJ, Kelso JRM (1998) Does habitat creation contribute to
management goals? An evaluation of literature documenting freshwater habitat
rehabilitation or enhancement projects. Can. Tech. Rept. Fish. Aquat. Sci.. 2249: vi +
74p.
Smokorowski KE, Pratt TC, Cole WG, McEachern LJ, Mallory EC (2006) Effects on
periphyton and macroinvertebrates from removal of submerged wood in three Ontario
lakes. Can. J. Fish. Aquat. Sci. 63, 2038-2049.
Sobek AA, Schuller WA, Freeman JR, Smith RM (1978) Field and laboratory methods
applicable to overburdens and minesoil. U.S. Environmental Protection Agency,
Report EPA-600/2-78-054 p.47-50.
Sowa VA (2004) Aspects of sustainability following closure of the Steep Rock Iron
Mines, Ontario. Explor. Mining Geol. 12, 37-47.
Squyres SW, Knoll AH (2005) Sedimentary rocks at Meridiani Planum: Origin,
diagenesis, and implications for life on Mars. Earth Planet. Sci. Lett. 240, 1-10.
SRK (2007) Colomac-Zone 2 Pit: Summary of 2007 Results. Unpublished
memorandum.
Steinberg CEW, Schafer H, Beisker W, Bruggemann R (1998) Deriving restoration goals
for acidified lakes from ataxonomic phytoplankton studies. Rest. Ecol. 6, 327-335.
Stevens CL, Lawrence GA (1997) Estimation of wind-forced internal seiche amplitudes
in lakes and reservoirs, with data from British Columbia, Canada. Aquatic Sci. 59,
115-134.
Stevens CL, Lawrence GA (1998) Stability and meromixis in a water-filled mine pit.
Limnol. Oceanogr. 43, 946-954.
Stewart FM, Mulholland T, Cunningham AB, Kania BG, and Osterlund MT (2008)
Floating islands as an alternative to constructed wetlands for treatment of excess
nutrients from agricultural and municipal wastes – results of laboratory-scale tests.
Land Contamination and Reclamation 16, 25-33.
Stierle AA, Stierle DB (2005) Bioprospecting in the Berkeley Pit: Bioactive metabolites
from acid mine waste extremophiles. In Atta-Ur-Rahman (Ed.), Bioactive Natural
Products, Vol 32, Elsevier, Amsterdam, 1123-1175.
Sumer S, Pitts L, McCulloch J, Quan H. (1995) Alberta lake re-established after draining
to mine coal. Min. Eng. 47, 1015–1020.
Tadonleke RD, Sime-Ngando T, Amblard C, Sargos D, Devaux J (2000). Primary
productivity in the recently flooded 'Sep Reservoir' (Puy-de-Dome, France). J.
Plankton Research 22, 1355-1375.

94
Taylor EB (2004) An analysis of homogenization and differentiation of Canadian
freshwater fish faunas with an emphasis on British Columbia. Can. J. Fish. Aquat.
Sci. 61, 68-79.
Tempel RN, Shevenell LA, Lechler P, Price J (2000) Geochemical modeling approach to
predicting arsenic concentrations in a mine pit lake. Appl. Geochem. 15, 475-492.
Tipping E (1981) The adsorption of aquatic humic substances by iron oxides. Geochim.
Cosmochim. Acta 45, 191-199.
Tones PI (1982) Limnological and fisheries investigation of the flooded open pit at the
Gunnar uranium mine. Sask. Research Council, Special Publication No. C-805-10-E-
82. 70 pp.
Turner JS (1965) The coupled turbulent transports of salt and heat across a sharp density
interface. Int. J. Heat Mass Transport 8, 759-767.
U.S. Environmental Protection Agency (2006) Bioremediation of Pit Lakes (Gilt Edge
Mine) Annual Report 2005.
http://www.epa.gov/ORD/NRMRL/std/mtb/mwt/annual/annual2005/pit/giltedgemine.
htm Accessed January 5, 2007.
USGS (2000) USGS Ground-Water Software: MODFLOW-2000 Version 1.18.00.
http://water.usgs.gov/nrp/gwsoftware/modflow2000/
.
USGS (2007) PHREEQC (Version 2)-A computer program for speciation, batch-
reaction, one-dimensional transport, and inverse geochemical calculations.
http://wwwbrr.cr.usgs.gov/projects/GWC_coupled/phreeqc/
.
Vincent J (1995) Wetland plantings within the Mimico Creek estuary, Humber Bay park,
city of Etobicoke. In Kelso JRM, Hartig JH (Eds.) Methods of modifying habitat to
benefit the Great Lakes ecosystem. CISTI (Can. Inst. Sci. Tech. Inf.) Occas. Pap. No.
1, p. 133-138.
Vymazal J (1995) Algae and Element Cycling in Wetlands. Lewis Publishers.
Wakatsuchi M (1984) Brine exclusion process from growing sea ice. Contributions from
the Institute of Low Temperature Science, Series A 33, 29-65.
Walton-Day K (2003) Passive and active treatment of mine drainage. In Jambor JL,
Blowes DW, Ritchie AIM (Eds.) Environmental Aspects of Mine Wastes. Mineral.
Assoc. Canada, Short Course 31, 335-359.
Watzlaf GR, Ackman TE (2006) Underground mine water for heating and cooling using
geothermal heat pump systems. Mine Water Environ. 25, 1-14.
Wendt-Potthoff K, Frommchen R, Herzsprung P, Koschorrek M (2002) Microbial Fe(III)
reduction in acidic mining lakes sediments after addition of an organic substrate and
lime. Water Air Soil Pollut. 2, 81–96.
Wetzel RG (2001) Limnology: Lake and River Ecosystems, 3
rd
ed. Academic Press.
White WW III, Lapakko KA, Cox RL (1999) Static-test methods most commonly used to
predict acid-mine drainage: Practical guidelines for use and interpretation. In
Plumlee GS, Logsdon MJ (Eds.) The Environmental Geochemistry of Mineral
95
Deposits. Soc. of Economic Geologists, Reviews in Economic Geology 6A, 325-
338.
Wilkie MP, Simmons HE, Wood CM (1996) Physiological adaptations of rainbow trout
to chronically elevated water pH (pH equal 9.5): J. Experimental Zool. 274, 1-14.
Wollman K, Deneke R, Nixdorf B, Packroff G (2000) Dynamics of planktonic food webs
in three mining lakes across a pH gradient (pH 2-4). Hydrobiologia 433, 3-14.
Wolfe M, Norman D (1998) Effects of waterborne mercury on terrestrial wildlife at Clear
Lake: Evaluation and testing of a predictive model. Environ. Toxicol. Chem. 17, 214-
227.
Yokom S, Wilcox D, Axler R, McDonald M (1997) Recovery of a mine pit lake from
aquacultural phosphorus enrichment: Model predictions and mechanisms. Ecol. Eng.
8, 195-218.
Young KL, Woo MK, Munro DS (1995) Simple approaches to modeling solar-radiation
in the Arctic. Solar Energy 54, 33-40.

96
8. GLOSSARY OF TERMS AND ACRONYMS
ABA: Acid-Base Accounting. Usually used in reference to batch laboratory tests that
determine the acid potential or neutralization potential of a rock or soil sample.
Acid Mine Drainage: the oxidation of exposed sulfide minerals contained in waste rock,
mine tailings and exposed cut rock which subsequently reacts with water to create
acidic conditions.
Absorption: The process by which a dissolved solute passes through the cell wall into the
interior of a living organism. Absorption only occurs in living cells.
Adsorption: The process by which a dissolved solute attaches to the outer surface of an
inorganic or organic solid.
Aerobic: In close contact with air, especially referring to the presence of oxygen.
Algae: Photosynthesizing microbes that contain a cell nucleus (i.e., eukaryotes).
Includes both green algae and diatoms (algae with silica shells).
Alkalinity: The sum of titratable bases in a water sample. Usually determined by titration
of the sample with a dilute acid, such as H2SO4. The higher the alkalinity, the more
strongly buffered a water sample is with respect to a drop in pH.
AMD: Acid Mine Drainage
Ammonia: A chemically reduced form of nitrogen. Occurs mainly as NH
4
+
(ammonium
ion) at pH < 9.25, or as NH
3
(dissolved ammonia gas) at pH > 9.25.
Amphibolite: A metamorphic rock rich in the mineral amphibole.
Anaerobic: Isolated from air, especially referring to the absence of oxygen.
Anhydrite: CaSO
4
. Similar to gypsum, but with no water.
Anion: A negatively charged dissolved ion. Examples: Cl
-
, SO
4
2-
, HCO
3
-
Anorthite: Calcium rich plagioclase. Formula: CaAl
2
Si
2
O
8
Anoxia: the absence of oxygen. Relating to pit lakes it refers to conditions where oxygen
is completely absent from, at least in one area, of the lake.
Anthropogenic: resulting from the influence of human beings on nature, as opposed to
those occurring in natural environments
without human influences.
AP: Acid Potential. Usually determined by a total sulfide analysis, this is the acid-
generating potential of a rock or soil. Most commonly expressed in units of kg
CaCO
3
/ton.
Apatite: A common accessory mineral in many igneous rocks: Ca
5
(PO
4
)
3
(OH, F, Cl).
Human teeth are composed of hydroxy-apatite.
Aquaculture: the cultivation and rearing of aquatic organisms under controlled
conditions. Also referred to as fish farming.
97
Astrobiology: The scientific study of the origins of life on Earth and other planets.
Autotrophic bacteria: These are prokaryotic micro-organisms that use carbon dioxide
(CO
2
) as the source of carbon they need to grow and multiply.
Backfill: The process of filling an open pit mine with soil or rock.
Bacteria: Primitive micro-organisms (i.e., prokaryotes) that lack a cell nucleus.
Bacterial sulfate reduction: An important reaction involving the conversion of sulfate
(SO
4
2-
) to sulfide (H
2
S or HS
-
). This reaction does not occur at low temperature
without microbial catalysis, and requires a source of electrons, such as hydrogen gas
or low molecular weight organic compounds.
Basalt: A common volcanic rock composed of the minerals pyroxene, plagioclase, and
sometimes olivine.
Benthic: Referring to sediments or biota at the bottom of a water body.
Bicarbonate: HCO
3
-
, the main source of alkalinity in most natural waters.
Biaovailability: the rate and quantity of toxic substances that may occur in the body
Biocenoses: a group organisms interacting within a particular habitat that in turn form an
ecological community.
Biogenic meromixis: A sub-type of meromixis in which the total dissolved solids of the
bottom water layer is increased due to micro-biological reactions, such as iron
reduction.
BOD: Biological oxygen demand. A measure of the potential for aquatic biota to lower
the oxygen content of water in the absence of photosynthesis.
Brine exclusion: The process by which salt is excluded from growing bodies of ice. The
salt concentrates into high salinity brines which can descend by gravity through the
ice and underlying water column of a frozen lake.
Cation: A positively charged dissolved ion. Examples: Na
+
, K
+
, Ca
2+
, Mg
2+
Cementation: A metallurgical term referring to the precipitation of copper onto metallic
iron.
Chemocline: An interface separating two layers of water in a lake that have differing
chemical properties, such as salinity.
CO
2
: Carbon dioxide.
COD: Chemical oxygen demand. A measure of the potential for inorganic reactions to
lower the oxygen content of water.
Crenogenic meromixis: Development of a meromictic lake due to the influx of deep
groundwater of high salinity.
Cryogenic meromixis: A sub-type of meromixis in which the total dissolved solids of the
bottom water layer is increased due to brine exclusion and thermohaline convection
during ice formation.
98
Cyanobacteria: A sub-type of bacteria that photosynthesize. Formally referred to as
“blue-green algae”.
Dimictic: A lake that regularly turns over in the fall and spring of each year.
Double-diffusion convection: A rare phenomenon that can occur when the
monimolimnion of a water body is both warmer and saltier than the overlying water
column. Results in a characteristic “stair-step” vertical profile in salinity and
temperature.
Diagenesis: The process by which the texture and mineralogy of recently-deposited
sediment is slowly transformed by a combination of organic and inorganic processes.
Diopside: A green pyroxene mineral: MgCaSi
2
O
6
. In kimberlites, trace amounts of Cr in
the mineral gives an intense emerald green color.
DO: Dissolved oxygen (O
2
).
DOC: Dissolved organic carbon.
Eh: The difference in electrical potential (or redox potential) of a water sample with
respect to a standard solution. By convention, the universal standard for Eh
measurement is the standard hydrogen electrode. Most common units are volts (V) or
millivolts (mV).
Ectogenic meromixis: Development of a meromictic lake due to human manipulation,
e.g., by filling the lower portion of a lake with salty water, and then capping with
water of low salinity.
End use: The permanent, sustainable use that is selected for an environmental system –
such as a mining pit lake – after human intervention ceases. End uses can be
biological, recreational, socio-economic, or scientific.
Enstatite: Mg-rich pyroxene: MgSiO
3
EPA: The United States Environmental Protection Agency.
Epilimnion: The shallow layer of a lake.
Eutrophic: Characterized by high rates of algal photosynthesis and high nutrient load.
Often resulting in large fluctuations in dissolved oxygen concentration.
Eutrophication: Nutrient overload to a water body. Symptoms of eutrophication include
copious growth of algae and aquatic macrophytes and large seasonal and diurnal (24-
h) changes in dissolved oxygen concentration.
Evapoconcentration: The process by which the concentration of a dissolved substance
increases due to loss of water to evaporation.
Extremophile: A microbe that can thrive in conditions that would normally be
considered hostile to life, such as very high or very low pH, unusually high or low
temperatures, or extremely high salinity.
Facies: A geologic term, broadly equivalent to the word “sub-type”.
99
Felsic: A descriptive geologic term for a rock with a predominance of light-colored
minerals, such as feldspar and quartz.
Fermentation: A process by which microbes gain energy by breaking down large organic
compounds into smaller organic compounds. Methane (CH
4
) and carbon dioxide
(CO
2
) are common by-products.
Ferric iron: The most oxidized form of iron, with a valence state of +3. Often written as
Fe
3+
or Fe
III
.
Ferrous iron: A reduced form of iron, with a valence state of +2. Often written as Fe
2+
or
Fe
II
.
Flow-through lake: A lake that has groundwater entering on one side but exiting on the
other side.
Forsterite: A Mg-rich olivine mineral, Mg
2
SiO
4
. Common in mafic and ultramafic
igneous rocks, including kimberlite.
Gabbro: A coarse-grained, intrusive igneous rock with the same chemical and
mineralogical composition as basalt.
Graminoid: grasses and grass like plants.
Granite: A very common, coarse-grained, felsic intrusive igneous rock that is rich in the
minerals quartz, feldspar, and biotite.
Green rust: This is a metastable solid compound with an olive- to emerald-green color
that is rich in ferrous iron. Often forms when the pH of Fe
II
-rich mine waters is
rapidly raised, e.g., by addition of lime. Green rust is unstable in air and will quickly
oxidize to red rust, i.e., Fe
III
oxide.
Gypsum: CaSO
4
·2H
2
O. A common mineral in marine sediments, especially in
environments that have undergone evaporation.
HFO: Hydrous ferric oxide. Often poorly crystalline, with the approximate formula
Fe(OH)
3
. One of many red-brown ferric compounds that can form in AMD settings.
H
2
S: Hydrogen sulfide. A toxic gas formed by sulfate-reducing bacteria.
Heterotrophic bacteria: These are prokaryotic micro-organisms that need organic carbon
for their source of carbon to grow and multiply. Most heterotrophic bacteria respire
CO
2
as a waste product.
HMO: Hydrous manganese oxide. A poorly crystalline substance, usually dark brown or
black in color, that is a mixture of various oxidized forms of manganese, i.e., Mn
III
and Mn
IV
.
Holomictic: Referring to a lake that completely turns over, on average, at least once per
year.
Humidity cell: An apparatus used to simulate accelerated weathering of mine waste
materials.
100
Hydraulic conductivity: Usually abbreviated “K”, this is the ability of a rock or soil to
transmit groundwater in response to a hydraulic gradient. Although sometimes
referred to as permeability (k), the units are different. K has dimensions of velocity
(e.g., m/day) whereas k has units of area (e.g., cm
2
).
Hypabyssal: Referring to an intrusive igneous rock that crystallizes at relatively shallow
depth (e.g., less than 1 or 2 km below the ground surface).
Hypolimnion: The deep portion of a lake, which is normally separated from the
overlying epilimnion by a thermocline.
Hypoxic: Being severely depleted in dissolved oxygen.
Ilmenite: FeTiO
3
. A fairly common accessory mineral in igneous rocks.
Insolation: Flux of photons (sunlight) at a particular location.
IOB: Iron-oxidizing bacteria. Includes the common species Acidithiobacillus
ferrooxidans.
Jarosite: A common secondary ferric mineral in mine waste settings, this mineral is only
stable at low pH. The most common variety has the formula KFe
3
(OH)
6
(SO
4
)
2
,
although sometimes Na
+
or H
3
O
+
can substitute for K
+
.
K-feldspar: KAlSi
3
O
8
. A common rock-forming mineral, especially in granite.
Kimberlite: The main bedrock source of diamonds, this is a very rare igneous rock that
has a K-rich ultramafic composition. Often occurs as vertical “pipe-shaped” intrusive
bodies that can extend > 1 km in depth with a diameter of a few 100 m or less. Also
can occur as sub-horizontal volcanic layers.
Kinetic test: In the context of this report, a kinetic test is any laboratory or field
procedure that can be used to quantify changes in the chemistry of water in contact
with mine waste over a long time period (e.g., one year or longer). Humidity cells are
one common example.
L: Liter
Lacustrine: Referring to a lake.
Leachate: Water that has had its chemistry modified by contact with another substance,
such as solid mine waste.
Life History: typical pattern of stages followed by most individuals within a group of
organisms follow throughout their lives. Can refer to the history of changes that occur
within a taxonomic group.
Ligand: an ion, a molecule, or a molecular group that binds to another chemical entity to
form a larger complex.
Lignite: A solid biofuel with a density less than that of coal.
Lime: CaO, a chemical often used to raise the pH of mine water.

101
Limestone: A common sedimentary rock, mainly composed of the mineral calcite
(CaCO
3
).
Limnology: The science understanding the biological
, physical, chemical, geological and
hydrological
properties of inland waters.
Littoral: Referring to shallow water, as along the shoreline of many natural lakes.
Lotic: relating to, or living in, moving (flowing) water.
Macrophyte: A multicellular plant, usually with a vascular system to transmit water and
nutrients.
Macroinvertebrates: typically used to refer to aquatic organisms large enough to see
without a microscope.
Mafic: A descriptive geologic term for a rock with a predominance of dark-colored
minerals, such as pyroxene, olivine, and Ca-rich plagioclase.
Marcasite: A metastable form of FeS
2
, similar to pyrite but with a different internal
atomic structure.
Megacryst: Usually used in relation to volcanic or hypabyssal igneous rocks, a
megacryst is an individual crystal that is readily visible to the eye surrounded by a
matrix of very fine-grained, microscopic mineral matter.
Meromictic: Referring to a lake that is permanently stratified.
Mesocosm: A small-scale model of the real world that highlights one or another process
of interest. Usually used in reference to bench-top laboratory experiments that
attempt to simulate the natural environment.
Metalimnion: see Thermocline
Metalloid: A term that has a strict metallurgical definition, but is commonly used in the
environmental literature for the elements arsenic (As), antimony (Sb), selenium (Se)
and tellurium (Te).
Metastable: Referring to a compound that is thermodynamically unstable and should
convert to a more stable compound, but does so at a sufficiently slow rate that it can
persist for a significant length of time in the environment.
Methane clathrate: A solid composed of molecules of methane gas that are trapped in a
three-dimensional “cage” of water ice. Thermodynamically stable over a rather
narrow range of temperature and pressure, methane clathrates are most abundant in
subarctic soils (permafrost), or in oceanic sediment off continental shelves.
Methanogenesis: The process by which bacteria use hydrogen gas or another source of
electrons to convert organic carbon compounds into methane (CH
4
).
Methylation: The process – usually sped up by microbes – by which an inorganic
metallic compound is transformed into an organo-metallic compound, as in the
methylation of mercury (Hg
2+
) to form methyl-mercury (HgCH
4
+
).
mg/L: milligram per liter, also known as ppm, or part per million
102
Mixolimnion: That portion of a meromictic lake that mixes seasonally. Usually includes
the epilimnion and the hypolimnion, but not the monimolimnion.
Mole: An Avogadro’s number of atoms; i.e., 6 x 10
23
. The mass of one mole of a
compound is equivalent to its “gram formula weight”.
mol/kg: Also known as molal (m), this is a unit of concentration equivalent to the number
of moles of a dissolved substance per kg of water.
mol/L: Also known as molar (M), this is a unit of concentration equivalent to the number
of moles of a dissolved substance per liter of solution.
Monimolimnion: This term refers to the deepest layer of a stratified, meromictic lake
which – because of its high density – does not mix with the overlying water column.
Monticellite: Rare mineral of the olivine group with the formula: CaMgSiO
4
.
Morphometry: Exact details regarding shape and size.
Muscovite: A common rock-forming mineral, usually transparent or light brown, also
known as mica. Formula: KAl
3
Si
3
O
10
(OH)
2
.
Nanocrystalline: Solid particles that are less than roughly 0.1 µm in size. Such particles
can easily pass through most filters used in the field to collect “dissolved” water
samples.
Nitrate: NO
3
-
, an important nutrient for growth of algae and plants.
NP: Neutralization Potential. Usually determined by ABA analysis (e.g., Sobek test), this
is the acid-neutralizing potential of a rock or soil. Most commonly expressed in units
of kg CaCO
3
/ton.
NPR: Neutralization potential ratio. This is NP/AP, and is normally determined by ABA
analyses. NPR values < 1 indicate a high probability that the rock or soil will
generate acidic leachate during weathering.
Nutrient: A chemical compound essential for growth of algae or plants. Usually used in
reference to nitrogen (either as nitrate or ammonia) and phosphorous (usually as
phosphate), but may include other elements such as iron (Fe) and potassium (K).
Oligotrophic: Referring to a water body that is low in nutrient content and therefore has a
relatively low rate of primary production by plants and algae.
Olivine: A common rock-forming mineral in mafic or ultramafic igneous rocks. Usually
green in color. Formula: (Mg, Fe)
2
SiO
4
, but may also contain Ca, as in monticellite.
Peridotite: A rare ultramafic igneous rock rich in olivine and pyroxene.
Permafrost: Permanently frozen ground in land masses at high latitudes. Overlain by a
thin “active layer” which thaws seasonally. Can be up to 500m thick, but can also
thin or disappear beneath large bodies of water, such as a large river or lake.
Perovskite: This is a rare mineral found in some metamorphic rocks or mafic to
ultramafic igneous rocks, including kimberlite. Formula: CaTiO
3
.
103
pH: A common field measurement that quantifies whether a water is acidic (pH < 7) or
alkaline (pH > 7). pH is based on a log scale, and is defined as – log(aH
+
), where log
is base 10 and aH
+
is the chemical activity (molal concentration adjusted for non-
ideal behavior) of protons in the water sample.
Phlogopite: A Mg-rich (and Fe-poor) variety of biotite mica: KMg
3
AlSi
3
O
10
(OH)
2
.
Phlogopite is relatively rare but is found in K-rich mafic or ultramafic igneous rocks,
such as kimberlite.
Phosphate: PO
4
3-
. A common nutrient, the most common forms in natural waters are
H
2
PO
4
-
and HPO
4
2-
.
Photoreduction: The process by which sunlight causes a decrease in valence of a
transition metal, as in photoreduction of Fe
3+
to Fe
2+
.
Photosynthesis: The process by which plants, algae, and some forms of bacteria combine
carbon dioxide and water to form organic carbon molecules. This reaction requires
sunlight, and produces oxygen (O
2
) as a byproduct.
Phototroph: Any organism that requires sunlight to metabolize. Includes all plants and
algae and some forms of bacteria.
Pioneer Species: organisms which are the first to invade and establish in an area
subsequent to a disturbance (natural or human induced).
Plagioclase: A very common rock-forming mineral whose chemical composition can
vary from CaAl
2
Si
2
O
8
(anorthite) to NaAlSi
3
O
8
(albite).
Plankton: Microorganisms that are suspended in a water body. Includes phytoplankton
(algae) and zooplankton (microscopic animals).
Polymictic: Referring to a lake that turns over, on average, more than twice a year.
Polyprotic acid: A chemical compound that has more 2 or more protons, each of which
can be lost in sequence, depending on the pH of the water. Examples: carbonic acid
(H
2
CO
3
), phosphoric acid (H
3
PO
4
), sulfuric acid (H
2
SO
4
).
Porphyrytic: A textural term referring to an igneous rock with a bimodal grain size
distribution: i.e., coarse-grained phenocrysts in a groundmass of very fine-grained
minerals.
Potentiometric surface: This is the elevation to which groundwater will rise in a well or
bore hole. For shallow, unconfined aquifers, the potentiometric surface is equivalent
to the water table. For deep, confined aquifers, the potentiometric surface may be
above or below the elevation of the ground surface.
Primary productivity: A measure of the combined rate of carbon-fixation by aquatic
plants, algae, and autotrophic bacteria.
Put-and-take Fishery: the stocking of a waterbody with hatchery reared fish for
recreational fishing purposes. These are fisheries that are not self-sustaining and
require the continual addition of sport fish from an outside source (hatchery or fish
farm).

104
Pyncnocline: An interface between two water layers of varying density.
Pyrite: A very common sulfide mineral, FeS
2
. Weathering of pyrite can result in acid
mine drainage (AMD).
Pyroclastic: Literally “fire” and “fragment”, this is a sub-type of fragmental volcanic
rock that forms during an explosive eruption.
Pyroxene: A common rock-forming mineral in mafic or ultramafic igneous rocks. It is
usually green, brown or black in color, and can have a wide range in chemical
composition. Most pyroxenes are rich in Fe and Mg.
Quartzite: A rock mainly composed of quartz formed from the metamorphism of
sandstone.
Reactive waste: In the context of this report, reactive waste refers to any solid waste
product generated during the mining process that has the potential to generate
leachate of poor water quality.
Relative depth: A limnological term that can be thought of as the aspect ratio (i.e.,
depth/surface diameter) of the lake.
Respiration: A biological process in which an electron donor (usually an organic carbon
compound) is oxidized and an electron acceptor (usually O
2
, but sometimes nitrate,
sulfate, or metal compounds) is reduced. Most respiring organisms (including
humans) generate carbon dioxide (CO
2
) as a waste product.
Rhyolite: A common, usually light-colored volcanic rock with a felsic (silica-rich)
composition.
Sandstone: A sedimentary rock composed of cemented sand grains, usually rich in
quartz.
Schwertmannite: A common yellowish-brown to reddish-brown Fe
III
oxy-hydroxide
mineral that forms in AMD settings. It’s chemical formula can vary, but is
approximately Fe
8
O
8
(OH)
6
(SO
4
).
Serpentine: A green phyllosilicate mineral, (Mg
, Fe)
3
Si
2
O
5
(OH)
4
, that most often forms
during the weathering or low-temperature hydrothermal alteration of mafic or
ultramafic igneous rocks.
Sludge: The technical term for the solid precipitates and contained water that form due to
a rise in pH after lime treatment of AMD.
Spinel: This rare mineral, MgAl
2
O
4
, is a semi-precious gemstone and is sometimes found
in metamorphic or igneous rocks of unusual chemistry, such as kimberlite or
peridotite.
Stoichiometry: The number and relative proportion of reactants and products for a given
reaction.
Subaqueous: Taking place below the water line of a lake or river, as in subaqueous
water-rock interaction.
105
Sulfate: SO
4
2-
, a common anion in natural waters, especially AMD waters.
TDS: Total dissolved solids. Usually expressed in mg/L, this is the sum of the
concentrations of all dissolved substances in a water sample.
TSS: Total suspended solids. Usually expressed in mg/L, this is the sum of the
concentrations of all non-dissolved substances in a water sample.
Tailings: The waste produced after ore is crushed and processed in the mill. Tailings is a
slurry-like mixture of sand- or silt-sized rock particles and water, and can contain a
high percentage of pyrite.
Terminal lake: A lake that has no surface or sub-surface outlet for water.
Thermocline: An interface between two layers of water of contrasting temperature.
Thermohaline convection: General term for the movement of water in a lake or ocean
due to density gradients that are caused by differences in salinity and temperature.
With respect to pit lakes, salt exclusion during freezing of surface water could
possibly induce thermohaline convection.
Till: A type of glacial sedimentary deposit composed of boulders and gravel in a fine-
grained clay or silty matrix. Usually very poorly sorted.
Titration: A laboratory or field procedure in which a reagent is added dropwise to a
volume of water until a reaction of interest is complete. An alkalinity titration is one
example, in which dilute acid is added to a water sample until the pH is lowered to an
endpoint of pH 4.5.
Turbidity: A quantitative measure of the amount of suspended solid in a sample. Most
common units are NTU (nephelometric turbidity units).
Turnover: The process of vertical mixing of a stratified lake. May occur in spring and
fall when the temperature of the epilimnion passes through the density maximum of ~
4° C.
Ultramafic: A rock – usually igneous – that has a very low silica content and forms at
extremely high temperatures and pressures. Most ultramafic rocks have a mineralogy
dominated by olivine and/or pyroxene.
USGS: United States Geological Survey
Vadose zone: A hydrogeological term referring to the zone of unsaturation between the
water table and the ground surface.
Waste rock: Rock that is blasted and hauled away during excavation of a mine, but is too
low in grade to be processed in the mill. Unlike tailings, waste rock is not crushed.
Wedderburn number: A quantitative measure of the likelihood that a lake will turn over
which takes into account variables such as relative depth, vertical gradients in water
density, and wind speed.
Winterkill: A massive die-off of fish in a frozen lake, usually brought on by a severe
depletion in dissolved oxygen.
106
Xenolith: Literally “alien rock”, this is a fragment of country rock that fell into a molten
magma and is now frozen in place within an igneous intrusion.
ZOR: “Zone of Relaxation”. Rock immediately surrounding an open pit mine that may
undergo extensional cracking due to a drop in lithostatic pressure.
