
© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 1
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
AP
®
Biology
Daily Lesson Plans
(Sample Week of Lesson Plans)
This full-year curriculum includes:
• 142 sequential lesson plans covering the entire College Board curriculum including
laboratory skills and test preparation
• A pacing calendar, a materials list, student handouts and grading rubrics
• 100% hands-on learning so the teacher can provide a student-centered classroom
environment with no lecture
• Lab experiments, games, model building, debates, projects and other activities designed
to promote critical thinking
• A curriculum that exceeds all the expectations of the AP College Board Redesign
for 2012
Please visit our website at www.CatalystLearningCurricula.com to download additional sample
lesson plans or to place an order.
AP
®
is a registered trademark of the College Board, which was not involved in the production of,
and does not endorse, this product.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 2
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
AP
®
Biology
Daily Lesson Plans Curriculum
Table of Contents
(with one week of sample lesson plans to follow)
I. Fostering Student-driven Learning in an AP
®
class
II. Year Calendar and Adapting to Class Schedules
III. Materials List
IV. Daily Lesson Plans
A. Daily Lesson Plans – Biochemistry – 15 class days
B. Daily Lesson Plans – Cell Biology – 34 class days
C. Daily Lesson Plans – Genetics – 28 class days
D. Daily Lesson Plans – Evolution – 16 class days
E. Daily Lesson Plans – Anatomy and Physiology – 26 class days
F. Daily Lesson Plans – Ecology – 19 class days
H. Review for AP
®
Biology Exam

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 3
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
AP* Biology Daily Lesson Plans
Biochemistry Unit
(Samples of Week One)
Day 1
I. Topic: What is Life?
II. Warm-up: 5 minutes
Tell the students to look on the board everyday as they enter to find
the warm-up question for the day. Tell them to start their notes for
the day with a response to this question (they do not need to write
the question in their notes).
Prior to class, write the warm-up question on the board or projected
on a screen. Today’s question is: What is the definition of the word
“life”?
III. Activity One: Is It Alive? 35 minutes
Objectives:
a) The learner will (TLW) evaluate their own definition of life and
compare it with the definitions offered by their peers and other
sources.
b) TLW get experience exploring their comprehension and discovering
misconceptions about a given subject through participation in a
Socratic discussion.
c) TLW get a taste of what is in store for them in this class with an
activity that piques their curiosity.
Materials:
1 potato; 1 mushroom; 1 plant; 1 candle; 1 digital clock; 1 photo of
a virus; 1 fish; 1 package of bread yeast in warm sugar water (do
not mix yet); 1 small bowl of vinegar and baking soda (do not mix
yet).
Procedure:
1. Place the above items in a central location and ask the students to
examine them. Encourage the students to use all of their senses to gather
information about the items.
2. Tell the students you would like them to consider the question you are
about to ask, but you do not want it answered aloud yet. Ask, “Which of

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 4
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
these things is alive?” Then, mix the yeast with warm sugar water, and mix
the vinegar and baking soda, and ask the students to observe the reaction
that occurs in each bowl.
3. Let them consider the question for a minute (“Which of these things is
alive?”), then ask them to jot down a response on paper. Ask them to
back up their response with a short explanation.
4. Begin a Socratic discussion by posing questions that lead the students
from their present base of knowledge toward new ground. Remember to
let them take control of the discussion and openly debate one another.
Play devil’s advocate when possible and do not try to clarify everything in
absolute terms. Allow them thinking time and suggest they refer to their
textbook when they reach a stalemate or want a concrete answer. Some
guiding questions might include:
a. Which of these items is alive? (potato, mushroom, virus, fish,
yeast)
b. Why do you think they are alive?
c. How can I tell if something is alive? (the item has some of the
characteristics of life)
d. How can I tell if something used to be alive?
e. Are the criteria for what constitutes a living thing the same for all
scientists? (no; for example, viruses are not considered alive by
some scientists)
When new vocabulary terms are introduced ask, “How would you define
the word _____?” so that the students can think about what the word
means and/or debate its meaning.
5. At appropriate times, jot notes on the board. In order to have a more
student-centered learning experience, pick a student to be the note-taker
for the period or have each student who formulates a clear idea go to the
board and add it to the class notes. Keep track of the students’
conclusions and/or contradictions—not everything on the board has to be
correct; you can put question marks next to ideas that are not agreed
upon or you can list exceptions that are mentioned during the discussion.
Some ideas that you may want to cover:
• a list of the characteristics of life
• examples of things that don’t appear to be alive but are
• examples of things that appear to be alive but are not
• a clear definition of “life”
• definitions of any terms that come up, even if they seem simple (ex:
metabolism, growth, organization, cells, evolution, homeostasis);
encourage students to clarify new words by coming to a consensus
on their meaning
6. Once the students feel they have settled on a definition of life, ask the
students how life on earth might have begun. After a few minutes of
discussion, ask the students to break into pairs, or larger groups as
needed, so that each team of students has an electronic device with which

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 5
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
to access the Internet. Ask the groups to each explore one current
scientific hypothesis of how life on earth may have begun: the RNA world
hypothesis; metabolism first hypothesis; the hypothesis that life was
brought to earth on a meteor from another location (aka panspermia); the
hypothetical origin of life in deep sea vent reactions; lightening bolt striking
elements and small molecules in pools of sea water based on results from
Miller-Urey experiments; clay particle origins based on Graham Cairns-
Smith experiments; etc.
7. When the students are ready, have groups that explored different
hypotheses join up and share what they have found. Use the remainder
of the time set for this activity for discussion and debate.
Special note: Allow 5 minutes for steps 1-3, then begin the discussion. Keep the
discussion lively, but let the students debate one another instead of looking to
you for concrete answers. Play devil’s advocate and throw challenging questions
into the discussion. Alert them to exceptions to their conclusions, such as the
fact that mature red blood cells do not have a nucleus or DNA; the sugar
transporting cells of the phloem in a tree (sieve cells) also have little to no
organelle and are supported with nutrients and ATP provided by a neighboring
companion cell, many cells such as algae and muscle cells can be multi-
nucleate, etc. Do not verify conclusions, but instead hint that their textbook
would be a good place to look for support for their arguments. Keep the
discussion portion under 25 minutes, using a timer, so that you stay on schedule.
IV. Activity Two: Introduction to AP Biology 10 minutes
Objectives:
a) The learner will (TLW) explore the direction of this course and their
role in the journey.
b) TLW learn what is expected of them and what can be expected
from their teacher.
c) TLW set goals and map out a strategy for achieving them.
Materials:
One “Goal Setting for AP Biology” handout for each student.
Procedure:
1. Let your students know that this is a college-level class and it will be
taught the way college courses are.
2. Inform them of what you will expect from them, for example:
a. They will perform work for this class every day.
b. They will be expected to complete all of their assignments on time.
c. They will be expected to come to you with questions for clarification
and help whenever needed.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 6
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
3. Let them know what they can expect from you, for example:
a. You will give them assignments that fuel their curiosity and help
them understand and remember key concepts.
b. You will guide them through difficult concepts with examples,
laboratory experiments or demonstrations, activities, games and
other learning adventures.
c. You will provide access to the fascinating world of modern science
by giving them the opportunity to talk to guest speakers, go on field
trips and experience real-world scientific research.
d. You will prepare them to take and pass the AP Biology Exam,
which will give them one year of college credit in laboratory
science.
4. Give the students a copy of the year calendar, so they can see what topics
this course covers.
5. Let them know what to expect from the curriculum, for example:
a. Daily homework, reading and note-taking
b. Weekly videos, labs and free response essays
c. Monthly lab reports or presentations
d. Tests on 2+ chapters every 2-4 weeks.
6. Ask the students if they are up for the challenge presented by this course.
Ask them what they would like to earn on the AP Exam. Have them write
that score on their goal sheet now.
7. Ask them to plan out how they are going to work toward their goal. Have
them write down how they will find the time to do 1-2 hours of work daily
for this course and when and where they will study each day.
8. Have them complete their goal sheet by writing out how they will motivate
themselves to maintain their study schedule.
9. Discuss what you could do to help them stay motivated. (It might help to
give them examples, such as minimal lecture time, interesting classes, no
“busy work”, help with study skills, audiotaping classes, use of colored
markers, silly songs created for mnemonic purpose, rewards such as
chocolate or candy, etc.)
10. Tell them that this goal setting handout is tonight’s homework assignment.
Ask them to complete the handout and have three people who can
support their study efforts and help them achieve their goals read it and
sign it.
HW: Ask the students to read the introduction to the biology chapters in their
textbook and take notes to turn in for a grade.
HW: Ask them to finish the goal setting handout to turn in for a grade.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 7
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Goal Setting for AP/IB Biology
The things I hope to gain from this class are (think of all the reasons you wanted to take this
course):
Primary goals:
1.
2.
3.
Secondary goals:
1.
2.
3.
The grade I would like to make on the AP/IB Exam is: __________
The AP exam is graded on a scale of 1-5. Scores of 3-5 earn you college credit in laboratory
science at some universities, with a score of 5 giving you the most college credit. The IB Biology
Exam is grade on a scale of 1-7. Scores of 6-7 are most likely to earn collge credit.
I will find the time to perform 1-2 hours of work daily for this course by doing the following:
1.
2.
I will study each day at (time) ________________ in (place) ________________.
I can help myself remember my goals and work toward them by:
1.
2.
3.
My teacher can help me obtain my goals by:
1.
2.
3.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 8
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
AP* Biology Daily Lesson Plans
Biochemistry Unit
(Samples of Week One)
Day 2
I. Topic: The Scientific Process
II. Warm-up: 5 minutes
Prior to class, write the following on the board: What are the steps
of the scientific process? Explain the purpose of each step.
III. Activity One: Scientific Method 30 minutes
Objectives:
a) The learner will (TLW) question the rigor of the scientific process to
understand why it is used in modern scientific research.
b) TLW debate the structure of several experiments in regard to their
scientific merit.
Materials:
One “The Scientific Process Critiqued” handout per student.
Procedure:
1. Ask students to work on the handout in pairs. Have them debate the
strengths and weaknesses of each experiment. Ask them to take notes as
they work.
2. After they finish, ask them to reconsider the warm-up question and write a
new response to it at the bottom of their handout.
3. Lead the class in a Socratic discussion. Some guiding questions might
include:
a. What is the purpose of the scientific process? (to test hypotheses
in a verifiable and credible manner)
b. What is the difference between a null hypothesis and a hypothesis?
(a null hypothesis is one that can be rejected—it essentially states
that the variable will have no effect on the outcome of the
experiment; null hypotheses are used in all scientific research so
that conclusions can be drawn that “reject” or “fail to reject” the null
hypothesis; see the Teacher’s Version of the handout for examples)
c. Considering the concept that nothing in science is ever “proven”,
but instead ideas are supported, explain why scientists test null
hypotheses instead of hypotheses. (because you can never have
enough support for a hypothesis to say that it is the sole

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 9
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
explanation of a phenomenon, but you can have enough data to
show that it is not the sole explanation of a phenomenon)
d. What constitutes a good scientific design? (repeatability, large
sample size, simple and elegant procedure, one variable while all
other factors are controlled, etc.)
e. What are the typical weaknesses of scientific experimentation?
(absence of one or more of the above-mentioned traits)
f. Why does sample size need to be very large? (to minimize
undetected variables or variables that cannot be eliminated)
g. Why does an experiment need a control? (to serve as a
comparison to measure against)
h. What is the ideal number of variables? (one per experiment)
i. Why are the steps so rigid? (consistency, repeatability, etc.)
j. What is the difference between inductive and deductive reasoning?
(Inductive - specific information leads to a general conclusion
Deductive - general information leads to a specific conclusion)
k. What is the difference between qualitative and quantitative data?
(qualitative is descriptive data, quantitative is numeric data)
l. Which is stronger? (quantitative)
IV. Activity Two: Introduction to the Free Response Essay 15 minutes
Objectives:
a) The learner will (TLW) understand what is expected on a Free
Response (FR) essay question, according to the AP Biology Exam
grading guidelines.
b) TLW learn to do preliminary work before beginning the written
essay.
Materials:
One copy of Free Response essay question #4 from the 2004 Form B
AP Biology Exam per student (this question, as well as the grading
rubric, can be printed from the www.apcentral.collegeboard.com
website).
Procedure:
1. Prior to class, go over the grading rubric for this and other free response
questions. Become familiar with how points are scored for most
questions. Learn the expectations for FR essays in terms of their
structure and content (for example: lists and outlines will not be graded;
venturing an incorrect idea will not count against a student, however they
will not receive points for incorrect ideas, etc.). Inform the students about
the Free Response portion of the AP Exam either by telling them about
the exam or by giving them an “Acorn” book with the exam information in it

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 10
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
(the pdf book of AP Biology Course information—with the picture of an
acorn on the front—can be downloaded at the
www.apcentral.collegeboard.com website).
The exam will contain two longer, multi-part Free Response (FR)
essay questions (typically broken into parts a., b. and c.) and six shorter
(single-part) essay questions that together count as 50% of the exam
grade. Ninety minutes are given to complete all six essays, which breaks
down to about 7 minutes with which to work on each short essay and each
part of the longer essays. Each of the longer essay questions is broken
into two, three or four parts that may require separate essays, calculations
or graphing, all to be completed within the suggested time frame.
In preparation for the essay part of the exam, students will write 1-5
practice FR essays per week during the school year under timed and
untimed conditions. Even if an essay assignment used to prepare the
student is untimed, students should note the time at which they begin and
finish writing the essay and set a timer for the number of minutes allotted
for completion of the essay, so that they become aware of how much time
they are using.
All FR essays must be neatly written, complete and to the point,
with three supporting facts, examples or statistics given for each main
point. All scientific terminology should be defined and all work must be in
complete sentences—outlines and lists do not earn points.
Students should always make a preliminary outline or mind map of
their main points and each of the three supporting details for each point to
be sure they have an answer to the question. On the actual AP Biology
Exam, the students will be given 10 minutes of reading time in which they
can outline their response. The reading period is intended to give
students time to organize their thoughts. For this class, preliminary work
in the form of an outline or notes or some other incomplete response may
be graded, but on the exam preliminary work doesn’t count. Only
complete sentences written in the test booklet are graded.
2. Give the students a copy of the grading rubric that goes with this exam
question so they can see how points are given only for specific, detailed
responses.
3. Discuss what types of information are considered specific enough to earn
points. For instance, if a student writes “The organisms in the Kingdom
Fungi are different from the organisms in the Kingdom Plantae because
they obtain their food in different ways,” they are not likely to receive any
credit. A more specific response would state “Organisms in the Kingdom
Fungi are heterotrophs that cannot make their own food, while organisms
in the Kingdom Plantae are autotrophs that are able to synthesize organic
molecules from inorganic molecules”. Let the student know that the essay
questions are open doors that allow them to show the grader all that they
know about a subject area. Remind them continuously throughout the
year to earn points for their answers by:

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 11
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
a. using scientific terms correctly, in context (define terms using a
supporting clause if the word is new to them this year)
b. describing specific case examples, and never using general
references (name one specific organism or a single part of the
anatomy or an example of a specific behavior, or whatever the topic
calls for)
c. never simply naming something, but instead always identifying and
describing something with at least a phrase or a separate
descriptive sentence
d. being sure to give an answer that responds directly to what the
question is asking (in this case, one molecular and one cellular
example are requested)
e. clarifying what potential essay question terms mean before you
take the exam, for instance:
i. What is the difference between “compare” and “contrast”?
ii. What would be expected for a question that asks you to
“design an experiment”?
4. Using FR question #4 for practice, help the students craft an explicit,
detailed answer that is properly supported.
Special note: Tell the students that for the first semester, they will be allowed to
use their textbook when answering FR questions because their background and
foundation information is limited. Later in the year, you will decide, on a case-by-
case basis, when they can and cannot use their textbook. You may want to give
FR questions as pop quizzes whenever there is extra class time. For the first
semester, you should grade the students’ preliminary outlining and mind mapping
(giving credit for important points and supporting facts) and their final paragraph
response if development of the essay to that level has been assigned. Even if a
response is incorrect, the students will get the message that the time and effort
put into planning their response is worthwhile.
HW: Ask the students to finish FR essay question #4 from Exam 2004 Form B.
HW: Make 5 copies of the Paper Atomic Models for each student on cardstock
paper. Ask the students to cut out the orbitals and nuclei and punch holes where
indicated for use during class tomorrow.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 12
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
The Scientific Process Critiqued
Read and discuss each scientific experiment. Analyze each experiment’s
strengths and weaknesses with your partner by answering the questions that
follow.
Experiment 1:
Five tomato plants of the same height were placed in the same size pots, in the
same type of soil and each was given the same amount of water. Each plant
was under a light bulb of the same intensity as the others but each light was of a
different color. Each day, the plants were given light (each its own color) for 12
hours and left in darkness for 12 hours. The height of each plant was measured
in centimeters at the end of each week for 10 weeks.
Week # →
1
2
3
4
5
6
7
8
9
10
Light color ↓
Yellow
4
5
6
7
8
9
10
11
12
13
Green
4
4
4
3
3
2
2
1
0
0
Blue
4
4
4
5
5
5
5
6
6
6
Purple
4
4
5
5
6
6
7
7
8
8
Red
4
5
6
7
8
9
10
11
12
13
a. What question is tested by this experiment?
b. Write two hypotheses and one null hypothesis for this experiment.
H
1
:
H
2
:
H
0
:
c. What is the variable?
d. What factors are held constant in the experiment?

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 13
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
e. Is there a control? If not, what control would you suggest?
f. Write two conclusions for this experiment.
1)
2)
g. Describe the strengths of this experiment.
h. Describe the weaknesses of this experiment.
Experiment 2:
Five soup cans were painted black and five cans were painted white. A quarter
liter of 24°C water was added to each can each morning at 8 a.m. and the
temperature of the water in each can was recorded in degrees Celsius at noon
each day for seven days.
Mon
Tues
Wed
Thurs
Fri
Sat
Sun
Black #1
45
37
40
41
32
35
40
Black #2
45
37
40
41
32
35
40
Black #3
45
37
40
41
32
35
40
Black #4
45
37
40
41
32
35
40
Black #5
45
37
40
41
32
35
40
White #1
41
33
36
37
28
31
36
White #2
41
33
36
37
28
31
36
White #3
41
33
36
37
28
31
36
White #4
41
33
36
37
28
31
36
White #5
41
33
36
37
28
31
36

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 14
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
a. What question is tested by this experiment?
b. Write two hypotheses and one null hypothesis for this experiment.
H
1
:
H
2
:
H
0
:
c. What is the variable?
d. What factors are held constant in the experiment?
e. Write two conclusions for this experiment.
1)
2)
f. Describe the strengths of this experiment.
g. Describe the weaknesses of this experiment.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 15
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Experiment 3:
A scientist wanted to determine if classical music helped people relax more than
rap music. She asked 1,000 20-year-old men and 1,000 20-year-old women to
participate in her experiment at the same time each day, in the same location
and under the same conditions. She had each person rest on a bed while she
played a classical music recording for 30 seconds and then she asked them to
describe how they felt. She would then repeat this procedure, playing rap music
instead of classical music. She alternated the type of music played first, but she
always used the same sample of classical music and the same sample of rap
music.
a. What question is tested by this experiment?
b. Write two hypotheses and one null hypothesis for this experiment.
H
1
:
H
2
:
H
0
:
c. What is the variable?
d. What factors are held constant in the experiment?
e. Describe the strengths of this experiment.
f. Describe the weaknesses of this experiment.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 16
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Experiment 4:
A scientist wanted to determine which language is the hardest to learn. He
created an experiment using 6,000 African Gray parrots as test subjects. The
parrots were left alone in a room with a tape playing all day and all night. On the
tape was the word “hello” repeated 100 times in a row for each of 20 languages.
Each day the scientist went into the room and checked to see if any of the
parrots had learned any of the languages.
a. What question is tested by this experiment?
b. Write two hypotheses and one null hypothesis for this experiment.
H
1
:
H
2
:
H
0
:
c. What is the variable?
d. What factors are held constant in the experiment?
e. Describe the strengths of this experiment.
f. Describe the weaknesses of this experiment.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 17
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
The Scientific Process Critiqued
Teacher’s Version
Read and discuss each scientific experiment. Analyze each experiment’s
strengths and weaknesses with your partner by answering the questions that
follow.
Experiment 1:
Five tomato plants of the same height were placed in the same size pots, in the
same type of soil and each was given the same amount of water. Each plant
was under a light bulb of the same intensity as the others but each light was a
different color. Each day, the plants were given light (each its own color) for 12
hours and left in darkness for 12 hours. The height of each plant was measured
in centimeters at the end of each week for 10 weeks.
Week # →
1
2
3
4
5
6
7
8
9
10
Light color ↓
Yellow
4
5
6
7
8
9
10
11
12
13
Green
4
4
4
3
3
2
2
1
0
0
Blue
4
4
4
5
5
5
5
6
6
6
Purple
4
4
5
5
6
6
7
7
8
8
Red
4
5
6
7
8
9
10
11
12
13
a. What question is tested by this experiment?
Does the color of light affect plant height over a 10-week period?
b. Write two hypotheses and one null hypothesis for this experiment.
H
1
: Ex: Plants will grow better in green light.
H
2
: Ex: Plants will grow better in red light.
H
0
: The color of the light will have no effect on plant growth rates.
c. What is the variable? The color of the light given to the plant.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 18
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
d. What factors are held constant in the experiment?
The type of plant, the soil, the size of the pot, the amount of
water given to the plant, the amount of time each plant was in the
light and in the dark.
e. Is there a control? If not, what control would you suggest?
No. A plant could be placed in full spectrum (white) light.
f. Write two conclusions for this experiment.
1) Ex: The plants in this experiment grew tallest in yellow/red light.
2) Ex: The plants in this experiment did not grow as tall in green light.
(Remind students to limit their conclusions; they should not make them too
broad, since only one species was tested in this experiment.)
g. Describe the strengths of this experiment.
The question is testable, the other factors were controlled, there
was only one variable being tested, etc.
h. Describe the weaknesses of this experiment.
The sample size was too small.
Experiment 2:
Five soup cans were painted black and five cans were painted white. A quarter
liter of 24°C water was added to each can each morning at 8 a.m. and the
temperature of the water in each can was recorded in degrees Celsius at noon
each day for seven days.
Mon
Tues
Wed
Thurs
Fri
Sat
Sun
Black #1
45
37
40
41
32
35
40
Black #2
45
37
40
41
32
35
40
Black #3
45
37
40
41
32
35
40
Black #4
45
37
40
41
32
35
40
Black #5
45
37
40
41
32
35
40
White #1
41
33
36
37
28
31
36
White #2
41
33
36
37
28
31
36

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 19
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
White #3
41
33
36
37
28
31
36
White #4
41
33
36
37
28
31
36
White #5
41
33
36
37
28
31
36
a. What question is tested by this experiment?
What is the difference in water temperature for white or black cans?
b. Write two hypotheses and one null hypothesis for this experiment.
H
1
: Ex: The water in the white cans will be hotter than in the black
cans.
H
2
: Ex: The water in the black cans will be hotter than in the white
cans.
H
0
: The color of the can will not influence the temperature of the
water in the can.
c. What is the variable? The color of the can.
d. What factors are held constant in the experiment?
The size of the can, the amount of water, the time the temperature
was taken, etc.
e. Write two conclusions for this experiment.
(Answers will vary but should be limited to conclusions that rely
only on the data given. (Ex: The water in the black cans was 4
degrees warmer each day than the water in the white cans.)
f. Describe the strengths of this experiment.
The question is testable, the other factors were controlled, there
was only one variable being tested, etc.
g. Describe the weaknesses of this experiment.
None. The design of the experiment is fairly strong.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 20
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Experiment 3:
A scientist wanted to determine if classical music helped people relax more than
rap music. She asked 1,000 20-year-old men and 1,000 20-year-old women to
participate in her experiment at the same time each day, in the same location
and under the same conditions. She had each person rest on a bed while she
played a classical music recording for 30 seconds and then she asked them to
describe how they felt. She would then repeat this procedure, playing rap music
instead of classical music. She alternated the type of music played first, but she
always used the same sample of classical music and the same sample of rap
music.
a. What question is tested by this experiment?
Does classical music help people relax more than rap music?
b. Write two hypotheses and one null hypothesis for this experiment.
H
1
: (Answers will vary; ex: People will say that they are more
H
2
: relaxed when they listen to classical music.)
H
0
: The type of music a person listens to will have no effect on
their level of relaxation.
c. What is the variable? The type of music.
d. What factors are held constant in the experiment? The length of time
each person listens to the music sample, the place and position of their
body when they listen, the age of the men and women, etc.
e. Describe the strengths of this experiment.
Consistency of age and procedure, and a large sample size.
f. Describe the weaknesses of this experiment.
There are many: the scientist is not testing what they set out to test,
the length of time the music sample is played is not long enough to
allow an effect, the procedure is qualitative instead of quantitative,
the effects the scientist is hoping to observe are undefined, etc.
Experiment 4:

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 21
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
A scientist wanted to determine which language is the hardest to learn. He
created an experiment using 6,000 African Gray parrots as test subjects. The
parrots were left alone in a room with a tape playing all day and all night. On the
tape was the word “hello”, repeated 100 times in a row for each of 20 languages.
Each day the scientist went into the room and checked to see if any of the
parrots had learned any of the languages.
a. What question is tested by this experiment?
The scientist wants to determine which language is the hardest to
learn, but the question that is really being tested is: Which of 20
languages has the most difficult greeting for African Gray parrots to
learn?
b. Write two hypotheses and one null hypothesis for this experiment.
H
1
: (Answers will vary; ex: African Gray parrots will find it easiest
H
2
: to learn English.)
H
0
: The difficulty of the language will have no effect on which
language African Gray parrots learn first.
c. What is the variable? The language.
d. What factors are held constant in the experiment? The number of times
each greeting is repeated, the housing and care of the parrots, etc.
e. Describe the strengths of this experiment. The sample size.
f. Describe the weaknesses of this experiment.
There are many: The scientist is not testing what they set out to
test – the hypothesis, the greeting does not represent the language,
the language acquisition of parrots is not the same as language
acquisition in humans, the parrots may have a significant influence
on one another, etc.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 22
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
AP* Biology Daily Lesson Plans
Biochemistry Unit
(Samples of Week One)
Day 3
I. Topic: Atoms and Ions
II. Warm-up: 5 minutes
(Ask the students to take and grade their quiz before they begin the
warm-up. See instructions below.)
Prior to class, write the following on the board: Draw an atom and
label these parts: electron, proton, neutron, nucleus and orbital.
III. Quiz: Introduction to Biology Concepts 5 minutes
Special note: For this quiz and each one that follows, write a short, ten-question
quiz designed to see if the students are keeping up with reading and vocabulary.
Do not use the quizzes to test deeper comprehension such as analysis and
synthesis of concepts—these can be assessed during activities, homework
assignments and tests. Train the students to pick up a quiz upon walking in the
door while you check roll. They can take the quiz at their desks, then come up to
correct the quiz at your desk using a grade sheet or a hole-punched overlay.
Have each student show their quiz to you when they have finished grading it, so
you can record their score. They will keep the quiz and return to their desk to
begin the warm-up or the first activity.
Quizzes should be added to the students’ year-long calendar or they
should be pre-set for the year so that the students can be held responsible
without being reminded (for instance, my students know they will have a quiz
each Tuesday on the chapters that will be covered during that week). From this
point on, I will not include quiz prompts in the lesson plans, since each teacher
will need to customize this activity to best fit into their own schedule.
IV. Activity One: Building Atoms 20 minutes
Objectives:
a) The learner will (TLW) review some of the chemical elements by
building models of atoms.
b) TLW review basic chemistry vocabulary and concepts by modeling
changes in proton, neutron and electron numbers.
Materials:

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 23
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
For each student: one cut-out and hole-punched copy of the
“Paper Atomic Model” handout, 25-30 M&M’s or Skittles candies, 1
copy of the periodic table of elements; for each lab group: 3 paper
cups or plastic “zipper” baggies.
Procedure:
1. Prior to class, sort the candy by color and place into paper cups, bowls or
plastic zipper baggies. Place three colors of candy at each lab table.
2. Using the periodic table, one nucleus and one orbital, have each student
make a helium atom with neutrons in one color sitting in the punched
holes of the nucleus, protons in another color sitting in the punched holes
of the nucleus and electrons in a third color sitting in the punched holes of
the orbital. Ask the students to draw the atom they have modeled in their
notes and label its parts.
3. Before discussing aloud, ask each student to jot down in their notes what
this atom would be called if the proton, electron or neutron number
changed. Discuss their answers and correct any misconceptions. Below
are a few points and terms that would be appropriate to review at this
time:
a change in the number of protons = a new element
a change in the number of electrons = a charged ion
a change in the number of neutrons = an isotope
* discuss radioisotopes
4. Have the students build a hydrogen atom with their paper atomic models.
Ask them to make each of the above changes one by one, and take notes
on how the atom is affected by each change.
5. Discuss the number of electrons that can fit on each orbital and have the
students practice building and drawing C, N, O and F, using their cut-out
orbitals and nuclei.
6. Discuss electronegativity and ask the students to note trends of
electronegativity above each column on their periodic table.
(electronegativity increases from left to right across a row and decreases
from top to bottom down a column)
7. Begin a Socratic discussion as students make notes on their periodic
tables and in their notes. You might begin the discussion by asking the
students to make some observations about atoms and elements, or you
can ask them to tell you some of the main points that they are writing
down in their notes today. You may want a note-taker at the board as a
guide, or you could take your own notes on an overhead projector
transparency of the periodic table, to model note taking during the
discussion.
Suggested guiding questions for discussion:

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 24
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
a. How many electrons fit on each orbital? (2 on the inner “s orbital”
and eight on the next two “p orbitals”)
b. How does the proton number compare to the electron number?
(they are the same on an element if it is unaltered/uncharged)
c. How do you think the number of electrons on the outer orbital of
each atom relates to how stable the element is? (elements made
of atoms with full outer orbitals—or valence shells—are more stable
and less reactive; elements made of atoms with partially complete
valence shells are more reactive; the closer the atom is to having a
completely full or completely empty valence shell, the more
violently it reacts with other atoms)
d. Which elements are the most stable? (the noble gases to the far
right, in column VIII) Why? (because they have full outer orbitals)
e. Which elements are the most reactive? (the halogens, column VII)
V. Activity Two: Building Ionic Molecules 20 minutes
Objectives:
a) The learner will (TLW) review basic chemistry vocabulary and
concepts by making models of ionic bonding.
Materials:
Same as Activity One, but with 2 cut-out and hole-punched copies
of the “Paper Atomic Model” handout per student.
Procedure:
1. Ask the students to use the paper atomic models to build a fluorine atom
and a hydrogen atom. From this point on, the students will not need to
represent neutrons or protons in their models if they are cognizant of the
fact that these atomic particles are indeed present.
2. Discuss the stability of each atom as it is modeled and show how the
loss/gain of an electron will stabilize the atoms’ outer orbitals by making it
either completely full or completely empty. Have the students move an
electron from hydrogen to fluorine to form two more stable atoms (ions).
3. Discuss how and why these atoms have formed an ionic bond. Ask them
to draw a “before and after” diagram of their HF molecule and take notes
on the bonding of ionic molecules.
4. Ask the students to build LiCl, MgO and BeS, drawing “before and after”
diagrams of each until they feel comfortable with the concept.
5. Begin a Socratic discussion by asking what main points the students are
making in their notes. Suggested guiding questions for discussion:
a. In each of the molecules they built, which atom has become the
cation, which is the anion? (the cations are Li, Mg, Be; the anions
are Cl, O, S)

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 25
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
b. Which elements of the periodic table tend to become negative ions
(have the greatest potential for acquiring electrons)? (the atoms in
column VII) Why? (because they are only missing one electron
and they have the high electronegativity needed to steal one from
another atom)
c. Which elements of the periodic table tend to be positive ions (have
the weakest ability to retain their electrons)? (the elements to the
far left, in column I) Why? (because they have low
electronegativity and have only one electron on their outer orbital)
d. Which columns would combine well in order for both to be more
stable? (columns I and VII, columns II and VI)
e. Which elements on the periodic table tend to make ionic bonds?
(columns I and VII, columns II and VI)
f. How does electronegativity correlate with ionic bonding? (atoms
with high electronegativity are able to steal electrons most easily,
atoms with low electronegativity tend to have their electrons stolen
from them)
g. Why don’t all the elements of the periodic table form ionic bonds?
(because at some point an element is unable to steal enough or
give enough electrons in order to become stable because it does
not have enough electronegativity to steal as many electrons as it
needs or it has too much electronegativity to give away as many as
would be necessary)
h. What do atoms that cannot form ionic bonds do to become more
stable? Read tonight and find out—this will be the warm-up
question tomorrow.
HW: Ask the students to write a response to the Free Response essay question
on radioisotopes to be turned in tomorrow.
HW: Assign the chapters on chemistry review and tell the students to take notes
to be handed in for grading. Tell them that from now on, they will need to look at
the year schedule to see which chapter topics are assigned for each week.
Remind them that they are responsible for knowing the information covered in
the textbook and may be quizzed on this material at any time.
HW: Tell them that from now on, they will need to look at the year schedule to
see which video topics are assigned for each week. Remind them that they are
responsible for knowing the information covered in the videos and may be
quizzed on this material at any time.
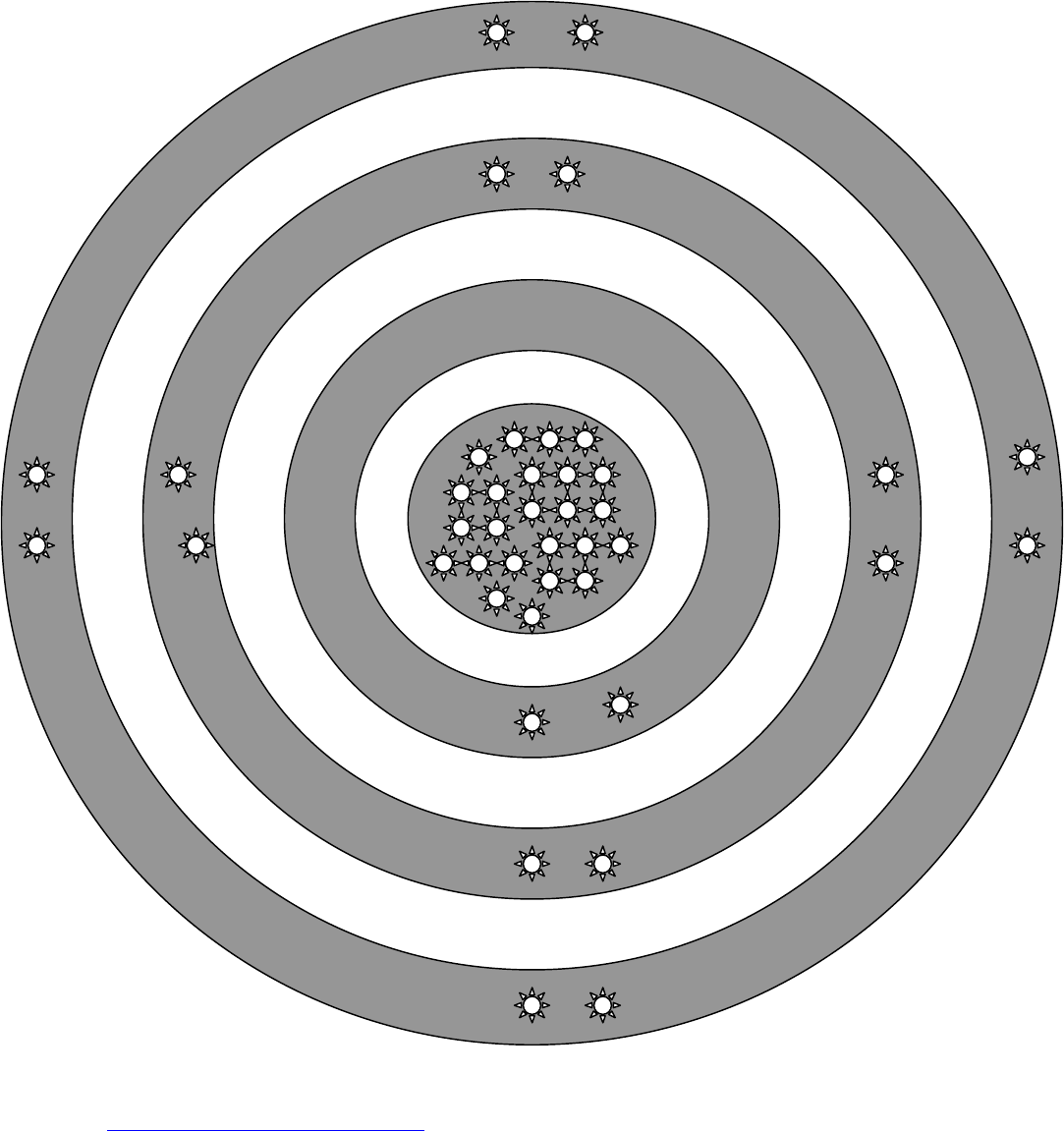
© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 26
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Paper Atomic Models
Cut out each gray ring and the gray circle in the center so that each ring is
separate from the others. Using a one-hole punch, punch out each star so that
there are 8 holes punched in the two large rings, 2 holes punched in the small
ring, and 24 holes in the center circle.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 27
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Free Response Essay
Radioisotopes
Suggested time: 22 minutes
Start time: ________ End time: ________ Total time used: ________
Directions: Answer all questions. Answers must be in essay form. Outline form
is not acceptable. Labeled diagrams may be used to supplement your response,
but in no case will a diagram alone suffice. It is important that you read each
question completely before you begin to write.
Radioactive elements are used for many purposes in science. Use your textbook
or other research sources to answer the following:
a) name three different radioisotopes
b) explain in detail how each of the radioactive isotopes you’ve listed
can be used in biology
** Try using a main-point-plus-three-supporting-details structure to organize and
write your essay. The main points will be the “answer” portion to the question
that was asked. In this case, the first main point will be naming a radioisotope.
The three supporting details might be additional information about that main
point, such as giving a definition of each of the terms used in the main point, a
more detailed description of the process or the idea of the main point, a statistic,
percentage or numerical detail that is associated with the main point or an
example of the point that is being made. In this case, the second main point will
be a description of how the named radioisotope is used in biology and the third
piece of supporting information will be a detail specific to that isotope, such as
naming the macromolecule it is most frequently bound to during scientific use.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 28
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Free Response Essay Grading Rubric
Radioisotopes
10 points total maximum
a) 1 point for naming a radioisotope
• give a point only if there is a description of at least one biological use
for the listed radioisotope
• give a maximum of 3 points for naming three radioisotopes
b) 2 points for describing how each isotope listed is used in biology
• give no points if the description is incomplete (for instance, if the
description is a one- or two-word answer)
• give 1 point for a simple statement of how the radioisotope is used
• give 1 point for an elaboration on how it is used
• a single isotope may have two completely different uses and such a
response would receive 4 points
• give a maximum of 4 points for any one isotope described; give a
maximum of 8 points for this section
A selection of example answers:
Statement of use Elaboration statement
H
3
Used as a
scintillation
marker
Can be attached to organic molecules and quantified by how
much light is given off as the alpha particles illuminate the
surrounding medium
P32
Used as a
radioactive
marker
Can be attached to the phosphate backbone of DNA or RNA
Used to track mRNA or find nucleic acids
C
14
Used to date
fossils
This isotope is added to organic molecules in constant
amounts when creatures are alive, so it can be used to
calculate how long a once-living thing has been dead,
depending on how much of the carbon has decayed to
nitrogen
O
18
Used to make
heavy water
Heavy water can be tracked through an organism during
experimentation; for instance, it was used to discover the
origin of the oxygen atoms added to sugar during
photosynthesis
S
Used as a
radioactive
marker
Can be attached to proteins on the disulfide bridges of most
polypeptides; for instance, it was used to make protein coats
of virus particles radioactive in the Hershey-Chase
experiment
I
Used in cancer
treatment
Can be used to fight cancer of the thyroid because it binds
with thyroid tissue and destroys the cells in this region during
radioactive decay
*This chart is not complete; there are other possible responses that can earn points. Also, realize
that in some students’ essays, answers included in the simple statement column above may be
found in the students’ elaboration statements, and vice versa.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 29
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
AP* Biology Daily Lesson Plans
Biochemistry Unit
(Samples of Week One)
Day 4
I. Topic: Covalent Bonding and Hydrogen Bonding
II. Warm-up: 5 minutes
Prior to class, write the following on the board: What do atoms that
cannot form ionic bonds do to become stable?
III. Activity One: Building Covalent Molecules 20 minutes
Objectives:
a) The learner will (TLW) review basic chemistry vocabulary and
concepts by modeling covalent bonding.
Materials:
Same as Activity One from yesterday, but with 5 copies of the cut-
out and hole-punched “Paper Atomic Model” handouts per student
(you can use about the same amount of candy as in Activity One
from Day 3, since neutrons and protons will be left out this time).
Procedure:
1. Ask students to make two hydrogen atoms. Ask if these atoms are likely
to form ionic bonds? (no, because they have equal electronegativity and
so cannot give or take an electron from the other) Ask the students what
the atoms can do to become stable? (form a covalent bond)
2. Have the students make a model of a covalent bond with their two paper
hydrogen models by sliding the atoms together and having them share the
electrons on their outer two orbitals (so that each is more stable with a full
valence shell).
3. Have the students take notes about this process, detailing how it happens
and why it happens.
4. Have the students practice covalent bonding on their own by making CH
4
and NH
3
. Stop to discuss the properties of covalent bonds and ask the
students to take notes, drawing diagrams of the atoms they have made.
5. Have them make a molecule with a double bond: O
2
and CO
2
6. Ask the students to divide into pairs or groups and practice making a
larger covalent molecule with C
2
H
6
and C
3
H
8
. Explain that to represent
larger molecules, scientists use organic model kits. Show them how to

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 30
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
make C
2
H
6
and C
3
H
8
or a double-bonded molecule like O
2
or CO
2
, using
an organic model kit.
7. Begin a Socratic discussion to draw out the main points of this activity:
a. How do the valence electrons influence molecular bonding? (the
number of open places on the valence shell determines the number
of bonds the atom can make)
b. How does electronegativity influence molecular bonding? (if there
is a large difference between the electronegativities of the two
atoms that are bonding, then the atom with greater electronegativity
can steal electrons from the atom with lower electronegativity to fill
its outer orbital; if the electronegativity of both atoms is equal or
nearly so, then the two atoms will form a covalent bond)
IV. Activity Two: Hydrogen Bonding 25 minutes
Objectives:
a) The learner will (TLW) review basic chemistry vocabulary and
concepts by modeling bonding between molecules.
b) TLW will compare and contrast ionic, covalent and hydrogen
bonding.
Materials:
Each student needs one copy of the “Comparing Molecular Bonds”
handout and an organic chemistry model kit containing the
following pieces: 3 carbon atoms, 2 oxygen atoms, 8 hydrogen
atoms, 1 halogen atom (F, Cl or Br), 10 single bonds and 4 double
bonds.
Procedure:
1. Have the students use the organic chemistry model kits to make CH
4
,
C
2
H
6
and C
3
H
8,
O
2
, CO
2
.
2. Ask them to take a moment to reflect and take notes on what these
models show.
a. What do the holes on each atom represent? (the number of
valence shell electrons that are missing for a full/stable outer
orbital)
b. How many bonds can H, O and C make? (H – 1, O – 2 and C – 4)
3. Ask each student to make two molecules of H
2
O and one molecule of H
2
,
O
2
, CH
2
F and CH
4
.
4. Discuss symmetry and the concept of polarity.
a. Which molecules are symmetrical? (H
2
, O
2
and CH
4
)
b. Which are polar and which are non-polar? (H
2
O and CH
2
F are
polar, H
2
, O
2
and CH
4
are non-polar)

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 31
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
c. How does symmetry correlate with polarity? (symmetrical
molecules are nonpolar; asymmetrical molecules are polar)
d. How does electronegativity correlate with polarity? (an atom with
higher electronegativity can draw the electrons to it unequally,
creating an unequal distribution of charge, without becoming a
charged ion)
5. Ask the students to arrange their two H
2
O molecules in the position in
space that is most likely according to the polarity of each molecule. This
is the position of hydrogen bonding. Have them draw two or three water
molecules in their notes and show hydrogen bonding with dotted lines
between negative and positive poles as below:
δ- δ+
δ+
δ+ δ+ δ-
δ-
δ-
6. Test the students’ understanding of hydrogen bonding by asking them to
compare each of the three types of bonding. Also, ask them to fill in the
comparison chart on the “Comparing Molecular Bonds” handout.
HW: Ask the students to finish the “Comparing Molecular Bonds” handout.
HW: If your students did not do the Free Response essay questions on
statistical analysis, assign those questions over the next few class periods to
check on their skills and knowledge on the application of statistics of biological
data:
FR essay for question #1 from the 2013 AP Biology Exam
FR essay for question #1 from the 2014 AP Biology Exam

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 32
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Comparing Molecular Bonds
1. Which type of bonding forms the strongest bond? ______________________
2. Which type of bonding forms the weakest bond? _______________________
3. Explain why hydrogen will form an ionic bond with fluorine but only forms
covalent bonds with carbon.
4. Which types of bonds occur between atoms? ________________________
5. Which type of bonds occur between molecules?________________________
6. What is the maximum number of atoms that can be bonded
...in an ionic bond? _______________
…in a covalent bond?______________
…in a hydrogen bond?____________
7. Draw an example of each type of molecular bonding below, using the
molecule of your choice:
a. ionic bond (draw a “before and after” diagram):
b. covalent bond
c. hydrogen bond

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 33
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Comparing Molecular Bonds
Teacher’s Version
1. Which type of bonding forms the strongest bond? ionic
2. Which type of bonding forms the weakest bond? hydrogen
3. Explain why hydrogen will form an ionic bond with fluorine but only forms
covalent bonds with carbon.
Fluorine only needs one electron to fill its outer orbital, so it has a very
high electronegativity. Fluorine can take the electron from hydrogen,
leaving both atoms charged. Carbon needs four electrons in order to fill
its outer orbital. Carbon’s electronegativity is too low for it to steal an
electron away, but it can share an electron with hydrogen to make both
atoms more stable.
4. Which types of bonds occur between atoms? ionic and covalent
5. Which type of bonds occur between molecules? hydrogen
6. What is the maximum number of atoms that can be bonded…
…in an ionic bond? two in the molecule,
∞
in the crystal structure
…in a covalent bond?
∞
…in an hydrogen bond?
∞
7. Draw an example of each type of molecular bonding below, using the
molecule of your choice: (Answers will vary.)
a. ionic bond (draw a “before and after” diagram):
b. covalent bond
c. hydrogen bond

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 34
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
AP* Biology Daily Lesson Plans
Biochemistry Unit
(Samples of Week One)
Day 5 – Extended Class Period
I. Topics: Physical Properties of Ionic, Covalent and Hydrogen Bonds
II. Warm-up: 5 minutes
Prior to class, write the following on the board: Describe two or
three ways molecules of the same type interact. (molecular
attraction or repulsion, polar and non-polar interactions, etc.)
III. Activity One: Comparison of Bond Properties 85 minutes
Objectives:
a) The learner will (TLW) review basic chemistry vocabulary and
concepts.
b) TLW experience how substances with different molecular bonds
may react in similar circumstances.
c) TLW observe how intermolecular bonding determines many of the
physical properties of a substance.
Materials:
For each lab group: 1 glass slide; 2 capillary tubes; 2 crucibles; 2
watch glasses; 1 Bunsen burner (or candles); 1 ring stand; 30g salt;
30g sugar; 10ml vegetable oil; 150ml distilled water; 1 hot plate; 1
conductivity meter; 2 Petri dishes; 8 paper clips; 4 dissection
microscopes; 1 mass balance; 1 four- or six-sectioned plastic
reaction tray; and each student will need one copy of the
“Comparison of Bond Properties and Intermolecular Bonding”
handout.
Procedure:
1. Prior to class, set up all the equipment that will be used so that the
students have time to perform all of the activities covered in the handout.
Also, make or bring in a model of a crystal lattice, or find a textbook photo
or a laser disk visual of one.
2. Lead the students in a discussion of predictions for the lab activities
(asking them to debate predictions with supporting arguments) before they
begin the experiments.
3. Ask the students to perform the first activity on the handout as a class.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 35
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
4. Discuss the results and explanations for the results. Stop at this point and
discuss the 3-D structure of ionic solids as crystal lattices. Show the
students the crystal lattice model, photo or laser disk visual, so that they
can see the intermolecular bonding that is the essential concept in today’s
lab. Discuss the intermolecular bonding of covalent molecules (polar and
non-polar) so that the students can compare the three types of
intermolecular interactions. Their discussions and their answers to most
of the Reflection Questions should center around intermolecular bonding.
5. Ask the students to continue performing the handout activities in lab
groups. If the students’ lab skills are not strong, this is a good time to
review basic skills by going through each procedure together as a class.
6. Ask the students to complete the Reflection Questions for homework.
Special note: If there is a limited number of lab equipment, you can set up six
stations, wherein each lab group moves from station to station. If the lab groups
do not perform the experiments simultaneously, as a class, I strongly suggest
you supervise the station where the students are performing the melting point
experiment (Experiment D) so that the students do not overheat the substances
with the Bunsen burners. If a group completes a station and is waiting for
another station to become available, the students can begin working on their
Reflection Questions.
HW: Ask the students to finish the “Comparison of Bond Properties and
Intermolecular Bonding” handout.
HW: Remind the students to look at the year calendar for reading and video
assignments.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 36
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Comparison of Bond Properties
and Intermolecular Bonding
Introduction:
You will use an ionic solid, salt, and a covalent solid, sucrose (sugar), in the first
three lab procedures. It is important to note the structure of each of these
substances.
Ionic Solid – Salt
Covalent Solid – Sucrose (Sugar)
In the final three lab procedures, you will use a polar liquid, water, and a non-
polar liquid, vegetable oil. It is important to note the structure of each of these
substances.
H H
O
Polar Liquid – Water

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 37
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Non-polar Liquid – Vegetable Oil
Experiment A: Geometric Crystal Structure
Purpose: To observe the shape of an ionic solid and a covalent solid.
Hypothesis:
I think the ionic solid will / will not display a regular and orderly crystal shape.
The reason I think this is…
I think the covalent solid will / will not display a regular and orderly crystal shape.
The reason I think this is…
Procedure:
1. Using a dissection microscope on high power and top lighting, observe the
crystal shape of an ionic solid (salt) and a covalent solid (sucrose/sugar).
2. Draw each substance accurately in the space designated at the top of the
page titled “Comparison of Physical Properties Data Chart”. Take the time
to draw one or two typical crystals in great detail rather than trying to draw
everything in your visual field.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 38
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Experiment B: Hardness of Ionic and Covalent Solids
Purpose: To test which substance is harder, the ionic solid or the covalent solid.
Hypothesis:
I think the ionic / covalent solid will be harder. The reason I think this is…
Procedure:
1. Using a dissection microscope on high power with top lighting, observe
the shape of the covalent solid in a Petri dish. Use your fingernail to crush
the crystals until they break, then observe them under the microscope
again to note their cleavage patterns.
2. Repeat the first step, this time using the ionic solid.
3. Make note of the hardness of each type of crystal in the Comparison of
Physical Properties Data Chart. Which solid was more difficult to crush?
Repeat the procedure if you have any uncertainty.
Experiment C: Conductivity of Ionic and Covalent Solids
Purpose: To test whether or not an ionic or covalent solution will conduct
electricity.
Hypothesis:
I think the solution with the ionic solid dissolved in it will / will not conduct
electricity. The reason I think this is…
I think the solution with the covalent solid dissolved in it will / will not conduct
electricity. The reason I think this is…

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 39
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Procedure:
1. Fill two beakers, A and B, with 100ml of distilled water. Add 20g of the
ionic solid to beaker A and add 20g of the covalent solid to beaker B.
2. Stir each with separate stirring rods until each solid is completely
dissolved in the solvent.
3. Using a conductivity meter or a set of alligator clips, a battery, a light and
conductivity wires, place both probes in beaker A to see if the solution in
the beaker is able to conduct electricity.
4. Record your results in the Comparison of Physical Properties Data Chart.
5. Rinse the probes in distilled water and then repeat the conductivity test for
beaker B.
Experiment D: Melting Point of Ionic and Covalent Solids
Purpose: To test which of the two solids has a higher melting point.
Hypothesis:
I think the ionic / covalent solid will have a higher melting point. The reason I
think this is…
Procedure:
1. Using a mass balance, place 1g of the ionic solid in one crucible and 1g of
the covalent solid in the other.
2. Place one crucible in the crucible holder of a ring stand and cover the
crucible with a watch glass.
3. Light a Bunsen burner and adjust the air vent until the flame is low and
steady (a candle can be used in place of a Bunsen burner).
4. Ask the timekeeper to begin timing the reaction at the same moment the
Bunsen burner is placed under the crucible in the ring stand platform.
5. Watch carefully as the sample heats. When the ionic solid begins to
brown or pop and sputter, stop the reaction and record the melting time.
When the covalent solid melts on the edges and turns brown, stop the
reaction and record the melting time.
6. Record both results in the Comparison of Physical Properties Data Chart.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 40
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Experiment E: Demonstration of Capillary Action
Purpose: To test the capillary action of polar and nonpolar liquids.
Hypothesis:
I think the polar / nonpolar liquid will have greater capillary action. The reason I
think this is…
Procedure:
1. Using a separate dropper for each, place three drops of a polar liquid on
one end of a glass slide and three drops of a nonpolar liquid on the other
end of the slide.
2. Using a capillary tube, gently touch the end of the tube to the drop of the
polar liquid and watch the results. Repeat the procedure with the same
tube several times until the results do not change.
3. Repeat step 2 using a new capillary tube and the nonpolar liquid.
4. Describe the results in the Comparison of Physical Properties Data
Section.
Experiment F: Demonstration of Surface Tension
Purpose: To test the surface tension of polar and non-polar liquids.
Hypothesis:
I think the polar / nonpolar liquid will have greater surface tension. The reason I
think this is…
Procedure:
1. Add half a centimeter of the polar liquid to one petri dish and half a
centimeter of the nonpolar liquid to the other petri dish.
2. Using a clean and dry paper clip, try to gently lay the paper clip on the
surface of each liquid using the surface tension for support. Retrieve the

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 41
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
paper clip, dry it and try again if you don’t do it on the first try. You should
be able to have the paper clip float on at least one of the liquids.
3. Record your results in the Comparison of Physical Properties Data Chart.
Experiment G: Demonstration of Solvent Ability
Purpose: To test the ability of polar and non-polar liquids to dissolve an ionic and
a covalent solid.
Hypothesis:
I think…
The reason I think this is…
Procedure:
1. Using a sectioned reaction dish, place 2ml of the polar liquid into two
sections and 2ml of the nonpolar liquid into two sections.
2. Using a mass balance, measure two 0.5g portions of the ionic solid and
two 0.5g portions of the covalent solid. Add 0.5g of the ionic solid to the
first polar section and the first nonpolar section. Add 0.5g of the covalent
solid to the second section of the polar liquid and the second section of
the nonpolar liquid.
3. Record the start time.
4. Gently swirl the reaction dish so that the liquid in each of the sections can
dissolve the solids.
5. Record the time it takes for each of the solids to dissolve in each of the
solvents.
6. Record your results in the Comparison of Physical Properties Data Chart.
**Note: The solids in some sections may never dissolve entirely.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 42
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Comparison of Physical Properties Data Chart
Crystal Shape of Ionic Solid Crystal Shape of Covalent Solid
Ionic and Covalent Bonds
Ionic Solid
Covalent solid
Hardness Test
Conductivity Test
Melting Point Test
Polar and Nonpolar Liquids
Polar liquid
Non-polar liquid
Capillary Action
Surface Tension
Solvent Ability:
Ionic Solid
Solvent Ability:
Covalent Solid

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 43
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Reflection Questions
Experiment A
1. Which solid has greater definition and regularity? ___________________
2. Which solid is a crystal lattice with intermolecular bonding? ___________
3. Draw the intermolecular bonds of the crystal lattice structure.
4. How would intermolecular bonding change the physical properties of a
substance?
5. Using your results from this test, make one inductive conclusion about the
geometric shape of ionic and covalent solids.
Experiment B
1. Which solid was harder to break?________________________________
2. Which solid has larger and more regular cleavage planes?____________
3. Explain why cleavage planes on molecules of one type of bonding would
differ in their hardness from molecules of another type of bonding.
4. Can you imagine a case in which an ionic solid and a covalent solid might
be equal in their hardness? Describe the molecular structure of these
(imaginary or real) example substances.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 44
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
5. Using your results from this test, make one inductive conclusion about the
hardness of ionic and covalent solids.
Experiment C
1. Which substance conducted electricity better?____________________
2. Explain what is happening to the molecules of the ionic solid as they are
dissolved in distilled water.
3. Explain what is happening to the molecules of the covalent solid as they
are dissolved in distilled water.
4. Use your answers from the two questions above to explain how electricity
is more easily conducted by one substance compared to the other.
5. Using your results from this test, make one inductive conclusion about the
conductivity of ionic and covalent solutions.
Experiment D
1. Which substance melted faster? ____________________________
2. Consider the molecular bonding of the substance in question 1 and use
what you know about bonding to explain why one sample melted at a
lower temperature than the other substance.
3. Using your results from this test, make one inductive conclusion about the
melting points of ionic and covalent solids.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 45
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Experiment E
1. Which liquid moved up the capillary tube easier? _______
2. Explain how this liquid moved up the tube without a vacuum or other force
being applied to the tube externally.
3. Explain the meaning of the word coalescence as it pertains to water.
Include a description of how polarity plays a role in coalescence.
4. Using your results from this test, make one inductive conclusion about
intermolecular cohesion of polar and non-polar liquids.
Experiment F
1. Which liquid has greater surface tension? _____________________
2. Using a diagram and a written description, explain why this liquid has
greater surface tension.
3. Using your results from this test, make one inductive conclusion about
intermolecular cohesion of polar and non-polar liquids.
Experiment G
1. Which liquid was a greater solvent? _____________________________
2. Using a diagram and a written description, explain how the electronegativity
of this molecule increases its solvent ability.
3. Discuss the role of water in the origin of life.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 46
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Comparison of Physical Properties Data Chart
Teacher’s Version
Crystal Shape of Ionic Solid Crystal Shape of Covalent Solid
Ionic and Covalent Bonds
Ionic Solid
Covalent solid
Hardness Test
Harder than sugar
Softer than salt
Conductivity Test
Conducts electricity
Does not conduct (or
conducts very little)
Melting Point Test
Takes much longer to
melt
Melts more quickly and
thoroughly
Polar and Nonpolar Liquids
Polar liquid
Nonpolar liquid
Capillary Action
Climbed tube easily
Did not climb tube
Surface Tension
Floated paper clip on
surface
Could not float paper
clip on surface
Solvent Ability:
Ionic Solid
Dissolved
Did not dissolve
Solvent Ability
Covalent Solid
Dissolved
Did not dissolve

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 47
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Reflection Questions
Experiment A
1. Which solid has greater definition and regularity? ionic solid, salt
2. Which solid is a crystal lattice with intermolecular bonding? ionic solid, salt
3. Draw the intermolecular bonds of the crystal lattice structure.
Each large chlorine molecule is
bonded with every other sodium
molecule that surrounds it on six
sides. Likewise with sodium
chloride.
4. How would intermolecular bonding change the physical properties of a
substance?
Intermolecular bonding allows for more bonds to hold the atoms in
place so that the melting points and hardness increase.
5. Using your results from this test, make one inductive conclusion about the
geometric shape of ionic and covalent solids.
Answers will vary but conclusions should focus on ionic solids being
more geometric and regular due to the crystal lattice structure dictated
by intermolecular bonding.
Experiment B
1. Which solid was harder to break? ionic solid, salt
2. Which solid has larger and more regular cleavage planes? ionic solid, salt
3. Explain why cleavage planes on molecules of one type of bonding would
differ from molecules of another type of bonding.
Because the intermolecular bonding of ionic solids gives them specific
properties.
4. Can you imagine a case in which an ionic solid and a covalent solid might
be equal in their hardness? Describe the molecular structure of these
(imaginary or real) example substances.
This might occur when a polar covalent solid forms many regular
intermolecular hydrogen bonds, allowing the molecule to be
geometrically arranged in space like a crystal lattice.
5. Using your results from this test, make one inductive conclusion about the
hardness of ionic and covalent solids.
Answers will vary but should focus on ionic solids being generally
harder than covalent solids.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 48
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Experiment C
1. Which substance conducted electricity better? the ionic solution
2. Explain what is happening to the molecules of the ionic solid as they are
dissolved in distilled water.
They dissociate and move freely as charged ions.
3. Explain what is happening to the molecules of the covalent solid as they
are dissolved in distilled water.
The molecules move away from each other as the water molecules
move between them, but the atoms of sucrose/sugar stay bonded
within the individual molecules.
4. Use your answers from the two questions above to explain how electricity
is more easily conducted by one substance compared to the other.
The ions are small and charged. Cations will move to the negative
probe and the anions will move to the positive probe, allowing a flow of
electricity, while the covalent molecule is only polar and so is less
reactive to the different charged probes.
5. Using your results from this test make one inductive conclusion about the
conductivity of ionic and covalent solutions
Answers will vary but should focus on ionic solutions being good
conductors.
Experiment D
1. Which substance melted faster? the covalent solid
2. Consider the molecular bonding of the substance in question 1 and use
what you know about bonding to explain why one sample melted at a
lower temperature than the other substance.
Because there are very few intermolecular bonds and the
intermolecular bonds are hydrogen bonds instead of additional ionic
bonds.
3. Using your results from this test, make one inductive conclusion about the
melting points of ionic and covalent solids.
Answers will vary but should focus on a generality that covalent solids
have lower melting points than ionic solids.
Experiment E
1. Which liquid was able to move up the capillary tube easier? __ polar__
2. Describe how this liquid moved up the tube without a vacuum or other
force being applied to the tube externally.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 49
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Because of intermolecular, hydrogen bonding, cohesion of molecules
to walls of the tube and adhesion of molecules to one another allowed
the water to climb up the tube spontaneously.
3. Explain the meaning of the word coalescence as it pertains to water.
Include a description of how polarity plays a role in coalescence.
Coalescence is the merging of water into droplets which is aided by
attraction of opposite poles in polar molecules.
4. Using your results from this test, make one inductive conclusion about
intermolecular cohesion of polar and nonpolar liquids.
Polar liquids are able to hydrogen bond, thus they have the properties
of cohesion and adhesion, while non-polar liquids do not hydrogen
bond and so do not have these properties.
Experiment F
1. Which liquid has greater surface tension? ___polar
2. Explain why this liquid has greater surface tension using a diagram.
(Diagram should show hydrogen bonds adhering molecules together.)
3. Using your results from this test, make one inductive conclusion about
intermolecular cohesion of polar and nonpolar liquids.
Polar liquids adhere to one another better than non-polar liquids due to
hydrogen bonding, thus polar liquids have greater surface tension than
non-polar liquids.
Experiment G
1. Which liquid was a greater solvent? polar __
2. Explain how the electronegativity of this molecule increases its solvent
ability, using a diagram and a written description.
Electronegative poles of water can move next to electropositive poles
of the solute and electropositive poles of water can move next to
electronegative poles of the solute to encourage the solid to dissolve,
whereas a non-polar liquid does not have poles and so cannot work its
way in between the molecules of the solute. (Any part of this response
may be in diagram form.)
3. Discuss the role of water in the origin of life.
Due to the properties of cohesion, adhesion, solubility, polarity, specific
heat capacity and other characteristics, water is included in all current
hypotheses of the origin of life on earth and is considered to be a
necessary requirement for life on other planets. Some type of solution
must have been the site for chemical reactions to have occurred

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 50
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
wherein elements meet and interact to form molecules and molecules
randomly meet and interact to form macromolecules or undergo
reactions. Because water has the properties that allow for these
actions to take place, it is thought that the origin of life occurred in an
aqueous solution.
This sample set of lesson plans is an excerpt from
AP* Biology Daily Lesson Plans
by Kristen Daniels Dotti
available through
www.CatalystLearningCurricula.com

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 51
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Special Topics Curricula $175 (per licensed user)
Incorporating Math and Statistics into AP/IB Science
(14 lesson plans covering 20-22 class periods)
AIDS: A Case Study Approach to Exploring the Immune
System, Viruses and Community Health
(20 lesson plans covering 28-32 class periods)
Diabetes: A Case Study Approach to Exploring Energy Use, the
Endocrine System, Cell Signaling and Membrane Transport
(22 lesson plans covering 30-42 class periods)
Hemoglobin: A Case Study Approach to Exploring Proteins, the
Cardiorespiratory System and the Evolution of a Gene Family
(24 lesson plans covering 32-44 class periods)
Full-year Curricula $570 (per licensed user)
AP
®
Biology Daily Lesson Plans
Full-year curriculum for AP*/IB Biology
Experimental Biology Daily Lesson Plans
Full-year curriculum for general, Pre-AP*/IB or Honors Biology using inquiry
methods
AP
®
Environmental Science Daily Lesson Plans
Full-year curriculum for AP*/IB or general Environmental Science
*"AP"College"Board"is"not"involved"in"the"production"or"the"endorsement"of"this"curriculum.

© Kristen Daniels Dotti 2015 AP
®
Biology Daily Lesson Plans pg. 52
www.CatalystLearningCurricula.com This is a sample set of a full year curriculum.
Billing Information: Shipping Information:
Name of Billing Contact Person: Name of Shipping Contact Person:
_________________________________ __________________________________
Address: Address:
__________________________________ __________________________________
__________________________________ __________________________________
Telephone: Telephone:
Email: Email:
Name of School: ___________________
Name of Licensed User(s): _______________Email of Licensed User(s): ____________________
How did you hear about Catalyst Learning Curricula Daily Lesson Plans? ________________
A curriculum license extends to only one teacher. Additional copies must be purchased for each
teacher who will be using the curriculum in a given school or school district. Please contact us
using the email address below to receive a discount for multiple user licenses.
Product Title: AP Biology, APES, or
Experimental Biology; or Name of the
Case Study, Game or Syllabus
Quantity (number
of users – one user
per license)
Format
Desired
(CD or
download)
Price
Shipping ($0 for download or $14/CD, $14 per game): _______
Taxes (6.75%-8.25% sales tax, depending on local tax rates, for NC residents only): _______
Total: _______
Payment Method:
Purchase Order Number: _____________________________
Checks should be made out to Catalyst Learning Curricula
Credit Card have no charge if conducted on the website, $22 charge by email or mail.
Card Number: ________________________________ Expiration date: ________
Name on card: ________________ 3-digit security code: ____ Billing zip code: _______
You may either email a picture of this completed form to
Kristen.Dotti@CatalystLearningCurricula.com or mail the completed form to:
Kristen Dotti, 59 Clemmons St., Asheville NC 28801 (Ph: 828-687-0807)
