
Over the past several years, the prevalence of human
disease caused by nontuberculous mycobacteria (NTM)
has increased. Whether the increase in cases is real or
whether more cases are being recognized remains unclear.
Despite a considerable increase in knowledge about NTM
infections, they still represent a diagnostic and therapeutic
challenge for several reasons: 1) pathogenic isolates may
be indistinguishable from contaminant or saprophytic iso-
lates; 2) timely and reliable identication of isolates may
depend on proper communication between clinicians and
laboratory staff; 3) lack of standardized susceptibility test-
ing makes adoption of tailored therapies unrealistic; and 4)
lack of treatment guidelines exposes patients to toxic drugs
and disappointing outcomes. Laboratory research and mul-
ticenter controlled trials are needed to improve diagnosis
and treatment of these infections.
T
he >120 recognized species of nontuberculous myco-
bacteria (NTM) share common features: 1) they are
facultative pathogens; 2) evidence of human-to-human
transmission is lacking; 3) some NTM species are ubiqui-
tous and others have more restricted distribution; 4) treat-
ment may be difcult and vary according to the involved
organism and disease site; and 5) pathogenesis is still un-
dened, depending on the interaction between the micro-
organism and the host’s immune system (1). About 90%
of cases involve the pulmonary system; the rest involve
lymph nodes, skin, soft tissues, and bones. Less frequently
Extrapulmonary Infections
Associated with Nontuberculous
Mycobacteria in Immunocompetent
Persons
Claudio Piersimoni and Claudio Scarparo
Emerging Infectious Diseases • www.cdc.gov/eid • Vol. 15, No. 9, September 2009 1351
Author afliations: United Hospitals, Ancona, Italy (C. Piersimoni);
and Santa Maria della Misericordia University Hospital, Udine, Italy
(C. Scarparo)
DOI: 10.3201/eid1509.081259
CME ACTIVITY
MedscapeCME is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity
to earn CME credit. This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation
Council for Continuing Medical Education through the joint sponsorship of MedscapeCME and Emerging Infectious Diseases. MedscapeCME
is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
MedscapeCME designates this educational activity for a maximum of 0.75 AMA PRA Category 1 Credits™. Physicians should only claim credit
commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certicate of par-
ticipation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content;
(3) take the post-test and/or complete the evaluation at http://www.medscape.com/cme/eid; (4) view/print certicate.
Learning Objectives
Upon completion of this activity, participants will be able to:
Diagnose and treat nontuberculous mycobacterial (NTM) lymphadenitis effectively•
Identify elements of NTM osteoarticular infections•
Treat NTM skin infections according to standards of care•
Describe infections with rapidly growing mycobacteria•
Editor
P. Lynne Stockton, VMD, MS, Copyeditor, Emerging Infectious Diseases. Disclosure: P. Lynne Stockton, VMD, MS, has disclosed no relevant
nancial relationships.
CME Author
Charles P. Vega, MD, FAAFP, Associate Professor; Residency Director, Department of Family Medicine, University of California, Irvine,
California, USA. Disclosure: Charles P. Vega, MD, FAAFP, has disclosed no relevant nancial relationships.
Authors
Disclosures: Claudio Piersimoni, MD; and Claudio Scarparo, MD, have disclosed no relevant nancial relationships.

SYNOPSIS
reported are central nervous system disease, keratitis, and
otitis media (1,2). We reviewed the epidemiology, clini-
cal features, diagnosis, and treatment of the most common
extrapulmonary diseases associated with NTM in immuno-
competent persons (2–5).
Lymphadenitis
Localized lymphadenitis most commonly affects chil-
dren; peak incidence occurs at 1–5 years of age (6). The
route of infection is hypothesized to be by way of the lym-
phatic vessels that drain the mouth and pharynx. The most
frequently isolated species is Mycobacterium avium com-
plex (MAC), followed by M. scrofulaceum, M. malmoense,
and M. hemophilum (7). However, a growing number of
previously unrecognized slow-growing mycobacteria have
been implicated with increasing frequency in reports of iso-
lated or microclustered cases (Table 1) (8).
Generally, NTM adenitis is an indolent disease; most
patients are otherwise healthy and have as their sole clini-
cal sign a chronic neck mass that does not respond to an-
timicrobial drug therapy. The disease is usually unilateral
and occurs in the cervical, submandibular, or preauricular
lymph nodes, although parotid and postauricular node in-
volvement has been reported. The nodes enlarge and may
rapidly soften and rupture, forming a draining sinus. Al-
though spontaneous regression has occasionally been de-
scribed, healing usually occurs by brosis and calcication.
Pyogenic and tuberculous adenitis are the most important
differential diagnoses.
Although a presumptive diagnosis of nontuberculous
mycobacterial adenitis can be made on the basis of clini-
cal history and physical examination, denitive diagnosis
depends upon the recovery of mycobacteria. Every effort
should be made to obtain material for culture and further
identication. Cultures of draining and ulcerated lesions, es-
pecially when swabs are used for specimen sampling, have
been shown to give a lower diagnostic yield than do needle
aspirates (9) or tissue biopsy samples. For recovery of NTM,
use of a liquid medium or radiometric growth detection are
regarded as the standard. Moreover, in children who have
not been vaccinated with M. bovis BCG, puried protein
derivative skin testing may be used as a surrogate test to di-
agnose chronic cervicofacial lymphadenitis (10). Histologic
appearance of necrotizing granulomatous inammation with
various degrees of caseation is also diagnostic.
Treatment of uncomplicated NTM lymphadenitis is
complete surgical excision. Incision and drainage are dis-
couraged because they usually lead to sinus tract formation
with chronic discharge. In a recent well-designed trial in-
cluding 100 children with culture- or PCR-conrmed diag-
noses, surgery was more effective than chemotherapy; cure
rates were 96% and 66%, respectively (11). Total excision
should be performed as early as possible to maximize re-
covery of the causative agent, to prevent further cosmetic
damage, to prevent extensive spread and subsequently
more difcult excision, and to cure disease. For some pa-
tients, however, surgery is associated with substantial risk
either because of a discharging sinus or proximity of facial
nerve branches. For these patients, chemotherapy preceded
by a diagnostic biopsy sample is recommended. Chemo-
therapy should also be considered for patients in whom
lymphadenitis recurs after surgery or for whom all abnor-
mal tissue could not be excised. Recent data indicate ne-
needle aspiration as the preferred diagnostic technique for
patients with nontuberculous mycobacterial adenitis who
do not undergo surgical excision. The optimal chemothera-
peutic regimen and its duration are still undetermined, but
combination therapy including clarithromycin and a rifa-
mycin, either rifampin or rifabutin and/or ethambutol, may
be benecial.
Osteoarticular Infections
NTM infections involving the musculoskeletal system
are uncommon. However, when they do occur, both rapid-
and slow-growing species have been implicated in chronic
granulomatous infections involving tendon sheaths, bursae,
bones, and joints. These are usually acquired by direct in-
oculation of the pathogen from an environmental source or
a contiguous infection focus as a consequence of surgical
procedures, penetrating trauma, injuries, or needle injec-
tions. Most affected patients are immunocompetent, but
some mycobacterial species, such as M. chelonae and M.
hemophilum, are almost entirely recovered from patients
with serious underlying diseases (HIV infection, immu-
nosuppressive therapy, or blood disorders) (4). In a recent
study of vertebral osteomyelitis caused by NTM (12),
various degrees of immunosuppression were found in 17
(51.5%) of 33 patients. From the 31 patients with spinal
infection studied, the following NTM species were recov-
ered: MAC (n = 13), M. xenopi (n = 7), M. fortuitum (n =
1352 Emerging Infectious Diseases • www.cdc.gov/eid • Vol. 15, No. 9, September 2009
Table 1. Less frequently encountered mycobacterial species
recovered from immunocompetent persons with lymphadenitis*
Mycobacterium sp.
No. cases
reported
Identification method
M. bohemicum
3 GS
M. celatum
1 RH, HPLC, GS
M. genavense
1 RH, GS
M. heckeshornense
1 GS
M. heidelbergense
1 GS
M. interjectum
4 GS
M. lentiflavum
5 HPLC, GS
M. palustre
1 GS
M. parmense
1 HPLC, GS
M. simiae
4 RH, HPLC, GS
M. triplex
4 GS
M. tusciae
1 HPLC, GS
*Most persons were children. GS, gene sequencing; RH, reverse
hybridization; HPLC, high-performance liquid chromatography.
Extrapulmonary Infections and NTM
5), M. abscessus (n = 3), M. kansasii (n = 1), M. simiae (n =
1), and unidentied NTM species (n = 1). M. hemophilum
also causes NTM osteomyelitis infections in bone marrow
and solid organ transplants (13). The hand and wrist are the
most frequently reported sites of NTM tenosynovitis be-
cause of their abundance of synovial uid and tissue com-
bined with a higher probability of penetrating injury. Most
frequently, M. marinum and M. kansasii are involved; less
frequently, M. avium complex, M. szulgai, M. terrae, M.
fortuitum, M. chelonae, M. abscessus, M. malmoense, and
M. xenopi are found (14).
Clinically, osteoarticular infections caused by NTM are
indistinguishable from tuberculosis-associated infections.
Signs and symptoms such as localized pain (with or without
neurologic impairment), joint stiffness and swelling, low-
grade fever, sweating, chills, anorexia, malaise, and weight
loss have been reported (Table 2). On rare occasions, sup-
puration followed by extensive necrosis of the synovial tis-
sue may occur (3), although in more severe cases infection
may extend to the periosteum and lead to osteomyelitis (3).
The clinical course of the disease is typically protracted;
average time from onset of symptoms to diagnosis may be
as long as 10 months. To prevent severe tissue destruction
and neurologic disorders, prompt and accurate diagnosis is
essential. Diagnosis relies on clinical suspicion and must
be considered for patients with increasing musculoskeletal
system signs, those with inammation after penetrating or
blunt trauma, and those with underlying risk factors who
undergo a medical procedure. Culture of synovial uid and
tissue biopsy are mandatory for denitive diagnosis and
identication of the causative agent. Computed tomogra-
phy and sonography may help guide percutaneous tissue
biopsy sampling of infected areas or diagnostic aspiration
of intraarticular uid (15). Histopathologic examination
has shown a spectrum of inammatory changes, including
granulomatous lesions with or without caseation (3).
Lack of the following have greatly limited develop-
ment of consensus guidelines for the treatment of mus-
culoskeletal infections caused by NTM: correlation be-
tween in vitro susceptibility testing and clinical outcome,
standardized antimicrobial-drug susceptibility testing for
most NTM species, and clinical trials comparing differ-
ent therapeutic regimens among an adequate number of
patients. Prolonged chemotherapy with an isolate-tailored
Emerging Infectious Diseases • www.cdc.gov/eid • Vol. 15, No. 9, September 2009 1353
Table 2. Clinical signs associated with osteoarticular infections caused by nontuberculous mycobacteria
Disease Affected site Mycobacterium spp.
Underlying diseases and risk factors
(no. cases reported)
Arthritis,
osteomyelitis
Thumb (interphalangeal
joint)
M. malmoense
Rheumatoid arthritis (1)
Arthritis Knee, ankle
M. xenopi
None* (6), invasive medical procedure (1)
Arthritis Multiple joints: wrist, knee,
finger, ankle, elbow,
vertebrae, shoulder
M. kansasii
AIDS (13), rheumatoid arthritis (3), systemic lupus
erythematous (2), renal transplant (2), polymyositis (1),
progressive systemic sclerosis (1), myelodysplasia (1),
none (26), localized trauma (10), steroid therapy (10)
Arthritis Knee
M. kansasii
Prosthetic joint (1)
Synovitis (carpal
tunnel syndrome)
Wrist
M. szulgai
No underlying disease, fish-tank cleaning (2)
Tenosynovitis Hand
M. intracellulare
None* (1)
Tenosynovitis Hand
M. chelonae
Penetrating injury (2), fracture (1),
Immunosuppression (3)
Tenosynovitis Hand, wrist M. avium complex Steroid injection (1), trauma (8), surgery (4)
Osteomyelitis Sternum, foot, elbow
M. wolinsky
Cardiac surgery (1), stepped on nail (1), open fracture (1)
Osteomyelitis Femur, tibia, calcaneus,
toe, elbow, sternum
M. goodii
Open fracture (4), stepped on nail (1), surgery (2),
penetrating trauma (2), puncture wound (1)
Osteomyelitis Wrist
M. scrofulaceum
Diabetes (1)
Osteomyelitis Hand, ankle, wrist
M. marinum
Fisherman exposed to aquarium (25), trauma (1), local
or systemic steroids (20)
Osteomyelitis
M. ulcerans
Traumatic injury (213)
Osteomyelitis Vertebrae
M. avium complex,
M. xenopi, M. fortuitum,
M. abscessus, M. kansasii,
M. simiae
Systemic lupus erythematous and treatment with
steroids (7), AIDS (4), interferon receptor defect (3),
carcinoma (1), renal failure (1), chronic granulomatous
disease (1), none* (16)
Osteomyelitis Vertebrae
M. xenopi
Vertebral disk surgery (58)
Osteomyelitis Tibia
M. conceptionense
Fracture (1)
Osteomyelitis Vertebrae
M. abscessus
Trauma to the back (1)
Osteomyelitis Vertebrae
M. avium complex
Trauma to the back (1)
Osteomyelitis Lower extremity, upper
extremity, vertebrae,
disseminated disease
M. hemophilum
AIDS (21), bone marrow or solid organ transplant (7);
AIDS plus solid organ transplant (1),
lymphoma (2), polycythemia vera (1)
*Otherwise healthy with no underlying disease or risk factor.
SYNOPSIS
drug combination associated with surgical debridement is
currently recommended for all musculoskeletal infections,
especially in patients with abscesses (12).
Skin and Soft Tissue Infections
Skin and soft tissue infections usually occur after trau-
matic injury, surgery, or cosmetic procedures, which may
expose a wound to soil, water, or medical devices occa-
sionally contaminated with environmental mycobacteria.
Although the epidemiology and clinical presentations of
NTM responsible for skin and soft tissue infections differ,
some species (MAC, M. kansasii, M. xenopi, and M. mari-
num) have been reported worldwide, whereas others (M.
ulcerans) have limited geographic distribution.
M. marinum causes diseases in many sh species and
is distributed worldwide. It is an opportunistic pathogen of
humans, in whom infection is infrequent and occurs by di-
rect injury from sh ns or bites or after cutaneous trauma
and subsequent exposure to contaminated water or other
sources of infection (shrimp, shellsh, frogs, turtles, dol-
phin, eels, and oysters). An increasing number of cases have
been reported from most countries with temperate climates
(1). Predisposing occupations and activities include sh-
ery worker, seafood handler, sh-tank owner, sherman,
pet shop worker, and water-related recreational exposure
(4). Consistent with the organism’s growth at low tempera-
tures, M. marinum infections are usually limited to the skin
and conned, with few exceptions, to 1 extremity. For sh-
tank owners, disease is often located in the hand or ngers;
for those who swim in pools, the elbow is affected for 85%,
followed by knee and foot (16). The incubation period is
usually <4 weeks but can be as long as 9 months. Signs and
symptoms of early infection are nonspecic, e.g., swelling
and pain followed by >1 skin lesions (17). At the inocula-
tion site, an erythematous or bluish papulonodular lesion
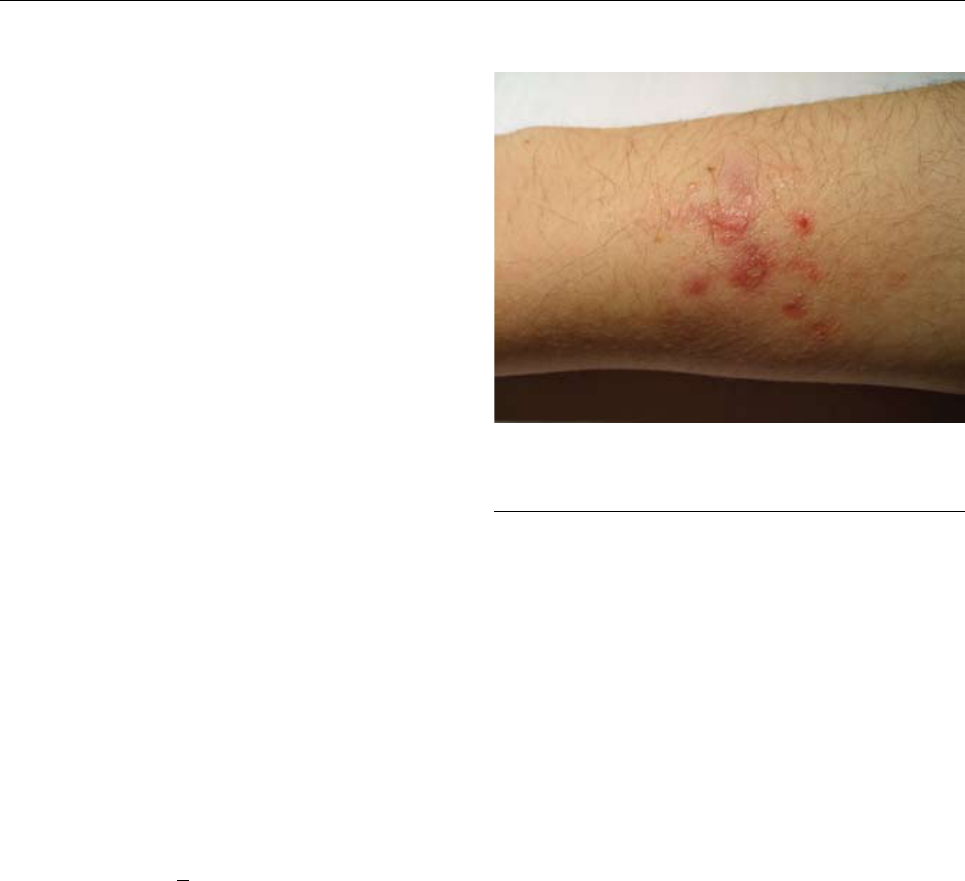
(≈0.5–3 cm) develops and slowly enlarges, becoming more
tender until it suppurates (18) (Figure). In ≈33% of patients,
M. marinum infection may spread to deeper structures (soft
tissues, tendons, and bone) (19), leading to extensive scar-
ring and varying degrees of functional impairment. Less
frequently, the disease extends from the inoculation site to
regional lymph nodes along the lymphatic vessels, mim-
icking the clinical appearance of cutaneous sporotrichosis
(20). Key elements for diagnosis of M. marinum infection
are a history of exposure to potential sources of infection;
a histopathologic appearance of granulomatous inamma-
tion but no caseation; and culture growth of M. marinum,
which strongly depends on incubation temperature. For lo-
calized skin lesions without a history of exposure to sh
tanks, swimming pools, or tropical sh, other NTM must
be considered (21).
Although presumptive identication can rely on a
few biochemical and phenotypic tests such as production
of photochromogenic pigment, negative nitrate reduction,
and positive tests for urease and Tween 80 hydrolysis, de-
nitive identication involves reverse hybridization tech-
niques, or, alternatively, high-performance liquid chroma-
tography analysis of mycolic acids and DNA sequencing
assays (22,23). In vitro susceptibility test results of M.
marinum clinical isolates have been reported extensively in
the literature. Only clarithromycin, minocycline, and ami-
kacin provide complete coverage (100% susceptibility);
doxycycline, rifampin, and trimethoprim/sulfamethoxazole
encounter different degrees of resistance (24). Drug-sus-
ceptibility testing of M. marinum isolates is recommended
only for patients who remain culture positive after several
months of therapy (25). Although a standardized regimen
for M. marinum disease is still undened, monotherapy with
doxycycline, minocycline, trimethoprim/sulfamethoxazole,
or clarithromycin should be limited to patients with mild
disease only. Clarithromycin combined with ethambutol or
rifampin is likely the best combination therapy. Treatment
with 2 agents should be continued for at least 1–2 months
after resolution of skin lesions. In addition, surgical treat-
ment (from mild debridement to amputation) may be re-
quired, especially when deep structures are involved (2).
M. ulcerans is the causative agent of Buruli ulcer, a
disease reported in >30 countries, mainly in tropical and
subtropical regions of Western and Central Africa but also
in Central and South America, Southeast Asia, and the
Western Pacic region (26). Currently limited knowledge
is mainly the result of a low number of reported cases and
inadequate surveillance. Recently, however, studies con-
ducted in some areas in which M. ulcerans is highly en-
demic have reported infection rates higher than those for
either tuberculosis or leprosy (27). Epidemiologic data on
detection of M. ulcerans DNA in aquatic insects (e.g., or-
1354 Emerging Infectious Diseases • www.cdc.gov/eid • Vol. 15, No. 9, September 2009
Figure. Mycobacterium marinum infection of the arm of a sh-tank
worker.

Extrapulmonary Infections and NTM
ders Odonata and Coleoptera) and snails, as well as in the
biolm of aquatic plants, strongly suggest that the organism
is associated with exposure to surface water involved in
environmental changes such as mining, deforestation, ag-
riculture, and hydraulic installations and that it is likely to
occupy a specic niche within aquatic environments from
which it is transmitted to humans by an unknown mecha-
nism (28). It is hypothesized that M. ulcerans reaches the
human dermis through a cut or wound contaminated with
water, soil, or vegetation. M. ulcerans is a unique species
able to produce a potent, virulence-associated toxin called
mycolactone, which prevents phagocytosis of live organ-
isms and induces tissue destruction by its cytotoxic and im-
munosuppressive properties. Although all age groups can
be affected, Buruli ulcer is more frequent among children
<15 years of age; the lower limbs (which are involved 3.2×
more often than the upper limbs) are the most frequently
affected sites (29). The incubation period varies but is gen-
erally <3 months (4). Buruli ulcer usually starts as a single
painless subcutaneous nodule or papule, which later moves
to form an ulcer with undermined edges (30). Spontaneous
healing usually takes 4–6 months and involves extensive
scar formation, resulting in severe deformity with joint
contracture, subluxation, atrophy, or distal lymph edema
(4). Sometimes tissue destruction may be so extensive that
amputation is unavoidable. In addition, dissemination to
distant sites can occur, especially in younger patients (<15
years). Multiple lesions represent the most severe form of
the disease; a high percentage of cases are osteomyelitis,
often leading to amputation or even death (29). Disabilities
are frequent, estimated for 25% to 58% of cases (31). Al-
though in M. ulcerans–endemic areas, diagnosis and treat-
ment are determined essentially by clinical appearances,
laboratory methods are available. These methods consist
of direct smear examination of specimens taken from the
ulcer edge or from tissue (≈40% sensitivity); culture incu-
bated at 29°C–33°C for 6–8 weeks (≈20%–60% sensitiv-
ity); histopathologic necrosis of subcutaneous tissues and
dermal collagen accompanied by a scant nongranuloma-
tous inammatory reaction embedding acid-fast bacilli
(≈90% sensitivity) (30); and PCR, a highly sensitive test
that can produce results within 2 days but is still conned
to reference and research laboratories (32). Case conrma-
tion requires at least 2 positive results from the above diag-
nostic tests (33). A standardized method for susceptibility
testing of M. ulcerans is not currently available (25). The
main treatment for Buruli ulcer is surgery. To ensure com-
plete removal of visibly affected tissue and to prevent re-
currence, excision should include a wide margin, including
healthy tissue. Although in early disease simple excision is
usually curative, in advanced disease wide and traumatiz-
ing excision is needed, followed by skin grafting and long
hospital stays. Although many antimicrobial agents have
demonstrated excellent in vitro and in vivo activity against
M. ulcerans clinical isolates, further studies are needed to
assess whether experimental susceptibility data will corre-
late with clinical outcome (33). A recent report stated that
rifampin plus streptomycin (1×/day for 4 weeks) and surgi-
cal excision inhibited the spread of infection and converted
early lesions (nodules and plaques) from culture positive
to culture negative (34). In edematous M. ulcerans disease,
the most rapidly progressive form, rifampin and strepto-
mycin have demonstrated a dramatically benecial effect.
Other treatments (e.g., topical phenytoin powder, topical
nitrogen oxides, and dressing plus triple-drug therapy [ri-
fampin, amikacin, and heparin]) are still being evaluated.
M. bovis BCG vaccination appears to offer some short-term
protection, especially against the most severe form of the
disease (33).
Cutaneous MAC disease occurs by direct inoculation
(trauma, surgery, injection) and is characterized by skin le-
sions such as ulceration, abscess with sinus formation, or
erythematous plaque with a yellow crusted base. The le-
sions are indolent, with little or no lymph node reaction or
systemic symptoms; some MAC skin infections resemble
Lupus vulgaris infections. Diagnosis requires a high index
of suspicion; a history of exposure to a potential source of
infection may be suggestive. For an accurate diagnosis,
histopathology, proper acid-fast bacilli identication, and
susceptibility testing are needed. A combination of exci-
sion (or surgical debridement) and chemotherapy is usually
required. Therapy is continued for 6–12 months and con-
sists of at least 3 drugs, usually clarithromycin, rifampin,
and ethambutol. Additional therapy with amikacin is some-
times included for 6 weeks (2).
Rapidly growing mycobacteria (RGM) are a complex
group of environmental pigmented and nonpigmented my-
cobacteria. Their optimal incubation temperatures range
from 25°C to 40°C, and they are characterized by a rapid
(within 7 days) growth rate on subculture. Organisms re-
sponsible for disease in humans belong to the M. fortuitum
group, the M. chelonae/abscessus group, and the M. smeg-
matis group; M. abscessus, M. fortuitum, and M. chelonae
are the most common species involved in cutaneous and
soft tissue infections. Able to survive in harsh conditions,
these organisms produce biolm in aquatic environments,
mostly in piped water systems from which large clumps
of mycobacteria are released into water and can be subse-
quently transmitted to humans (35,36). In addition, RGM
are resistant to sterilizing agents (2% formaldehyde and
glutaraldehyde), antiseptics (organomercurial compounds),
and other common disinfectants (37). Clinical manifesta-
tions of RGM disease largely depend on the immunocom-
petence of the infected person. Cutaneous and soft tissue
infections may appear as a single lesion in an immunocom-
petent person, usually after penetrating trauma or invasive
Emerging Infectious Diseases • www.cdc.gov/eid • Vol. 15, No. 9, September 2009 1355

SYNOPSIS
surgical procedure at the site of infection (M. fortuitum is
the predominant causative agent), or they may appear as
multiple or disseminated lesions, usually associated with
immunosuppressive treatments (especially long-term treat-
ment with steroids) or other immunosuppressive conditions
or concurrent illnesses (38). For the latter, M. chelonae and
M. abscessus are the predominant causative agents. Several
reported infections that occurred after traumatic, cosmetic,
or other medical procedures are summarized in Table 3.
In a large outbreak of RGM infection related to pedicures,
the median onset of signs and symptoms was 3 weeks, but
for some, signs and symptoms were delayed as long as 4
months after exposure (39). Histopathologic examination
of lesions showed suppurative granulomata and abscesses.
Areas of necrosis were typically seen, but caseation was
uncommon (21). Denitive diagnosis of clinically sus-
pected RGM soft tissue disease can be made by culture of
organisms from drainage material, aspiration uid, or tis-
sue biopsy sample. When NTM disease is suspected on the
basis of clinical signs, with any patient history, laboratory
staff should be alerted so they can use appropriate isola-
tion protocols (M. chelonae and some strains of M. absces-
sus are relatively heat intolerant and can be recovered by
primary isolation at 30°C). Identication of RGM at the
species level is of utmost importance because treatment
regimens and consequently clinical outcome are strongly
species related. Proper identication involves molecular
techniques (22) coupled with a few traditional biochemical
and phenotypic tests (use of the carbohydrates citrate, man-
nitol, inositol, and sorbitol; tolerance to 5% NaCl; nitrate
reduction; iron uptake; and 3-day arylsulfatase activity)
(35). Susceptibility testing performed by the broth microdi-
lution technique (25) is essential for choosing the most ef-
fective drug therapy and monitoring for the development
of mutational drug resistance (2). Most experts recommend
the use of specic antimicrobial drugs, given in combina-
tion to avoid the emergence of drug resistance. Treatment
for the M. fortuitum group may include amikacin, cefoxi-
tin, ciprooxacin, the newer quinolones gatioxacin and
moxioxacin, sulfonamides, and imipenem. Some degree
of susceptibility to doxycycline and clarithromycin has
been reported. M. abscessus strains are usually suscepti-
ble to amikacin, cefoxitin, imipenem, clarithromycin, and
azithromycin; M. chelonae are usually susceptible to amika-
cin, imipenem, tobramycin, clarithromycin, and sometimes
linezolid. Clarithromycin is generally the drug of choice
for localized disease caused by M. chelonae and M. absces-
sus (5,35). The duration of therapy is usually 4 months for
mild disease and 6 months for severe disease. Surgery is an
important complementary tool for treating these infections,
depending on disease severity and location.
Laboratory Diagnosis
Of all the currently described mycobacterial species,
≈60% have caused human diseases. For this reason, mod-
ern techniques for faster culture, identication, and drug
susceptibility testing are urgently needed in mycobacteri-
ology laboratories (22,25). In addition to the collection of
high-quality specimens, timely diagnosis of NTM disease
requires regular communication of clinical suspicion to the
laboratory staff because optimal recover of some fastidi-
ous species requires additional tasks. Routine techniques
include microscopy and culture; the latter should be per-
formed by using both liquid and solid media incubated at
1356 Emerging Infectious Diseases • www.cdc.gov/eid • Vol. 15, No. 9, September 2009
Table 3. Skin and soft tissue infections caused by rapidly growing mycobacteria
Type of infection or procedure Mycobacterium spp. Clinical findings
Posttraumatic wound infections
M. fortuitum, M. chelonae, M. abscessus,
M. wolinskyi, M. goodii, M. porcinum
Subcutaneous abscesses, cellulitis
Pedicures
M. fortuitum, M. chelonae, M. mageritense
Furunculosis
Subcutaneous, intraarticular, or
periarticular Injections
M. chelonae, M. abscessus
Subcutaneous abscesses, painful nodules,
multiple sinus tracts, joint infections, fever,
chills after injection
Acupuncture
M. abscessus, M. chelonae, M.
nonchromogenicum
Erythematous papules, nodules, ulcerative
lesions, abscesses, confluent plaques, draining
sinus tracts with discharge, wrist tenosynovitis
Cardiac surgery
M. peregrinum, M. fortuitum, M. fortuitum
(third biovariant) complex, M. wolinskyi, M.
goodii, M. abscessus
Sternal wound infection, endocarditis
Cosmetic surgery or other surgical
procedures: liposuction, liposculpture,
face lift, breast lift (reduction
augmentation), silicon injection
M. fortuitum, M. chelonae, M. chelonae, M.
porcinum, M. wolinskyi, M. goodii
Erythema, tenderness, nodules, skin
induration, subcutaneous or deep-tissue
abscesses, fever, malaise, multiple abscesses
along original suction tracts, draining sinus
tracts, local pain, swelling
Nipple piercing
M. fortuitum, M. abscessus
Asymptomatic nodules, tender nodules
Implanted with prosthetic material
M. abscessus, M. goodii
Abscesses
Pacemaker placement
M. fortuitum, M. abscessus, M. wolinskyi
Abscesses
Peritoneal dialysis catheter
M. abscessus
Abscesses

Extrapulmonary Infections and NTM
different temperatures (22). Although optimal recovery
for most clinically relevant mycobacteria is obtained at
35C°–37C°, some species (M. hemophilum, M. marinum,
M. ulcerans and some species of RGM) require a lower
incubation temperature to grow. For this reason, all clinical
specimens that may harbor the above species (skin, syn-
ovial uid, and bone) should be cultured at 28C°–30C° and
at 35C°–37C°. Use of conventional biochemical and phe-
notypic tests for the identication of NTM is currently dis-
couraged; more rapid and specic methods are favored, in-
cluding high-performance liquid chromatography analysis
of mycolic acids and commercial molecular assays. These
may use either in-solution hybridization (Accuprobe, Gen-
Probe Inc., San Diego, CA, USA) or solid-format reverse-
hybridization assays (line probe assays) (22).Both tech-
niques are specic, but the latter (in which amplication
precedes hybridization) is more sensitive, enabling iden-
tication in the early stage of bacterial growth. Finally,
gene (16S rDNA) sequencing is required for those species
that cannot be identied by the above systems (22). Care-
ful strategies should be recommended for using 16S rDNA
sequence analysis databases because public databases may
have wrong sequences and commercial ones tend to be un-
derdeveloped and outdated (23,40).
Conclusion
Although NTM cause a broad spectrum of human dis-
ease, data on incidence of NTM infections are still lacking,
mainly because of the absence of systematic epidemiologic
studies, standard case denitions, and accurate mycobacte-
rial identication. Furthermore, nonspecic clinical mani-
festations, lack of familiarity with these infections, and
inadequate laboratory services make denitive diagnosis
of NTM diseases often delayed or even impossible. Cor-
relation of in vitro susceptibility testing with the clinical
outcome, composition and duration of treatment regimens,
and use of surgery or other therapeutic approaches are still
undened for most NTM species involved in human dis-
eases. Laboratory research and multicenter controlled trials
are needed to improve diagnosis and treatment of extrapul-
monary NTM infections.
Dr Piersimoni is a clinical microbiology consultant at the
United Hospitals, Ancona, Italy. His primary research interest
focuses on diagnostic and clinical aspects of mycobacterial infec-
tions.
Dr Scarparo is a medical microbiologist and head of the
Clinical Microbiology Laboratory at the Santa Maria della Mise-
ricordia University Hospital, Udine, Italy. His primary interests
include the epidemiology, diagnosis, and treatment of mycobacte-
rial infections.
References
1. Falkinham JO. Epidemiology of infection by nontuberculosis myco-
bacteria. Clin Microbiol Rev. 1996;9:177–215.
2. Grifth DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C,
Gordin F. An ofcial ATS/IDSA statement: diagnosis, treatment, and
prevention of nontuberculous mycobacterial disease. Am J Respir
Crit Care Med. 2007;175:367–416. DOI: 10.1164/rccm.200604-
571ST
3. Marchevsky AM, Damsker B, Green S, Tepper S. The clinicopatho-
logical spectrum of nontuberculous mycobacterial osteoarticular in-
fections. J Bone Joint Surg Am. 1985;67:925–9.
4. Dobos KM, Quinn FD, Ashford DA, Horsburgh CR, King CH.
Emergence of a unique group of necrotizing mycobacterial diseases.
Emerg Infect Dis. 1999;5:367–78.
5. De Groote MA, Huitt G. Infections due to rapidly growing myco-
bacteria. Clin Infect Dis. 2006;42:1756–63. DOI: 10.1086/504381
6. Lai KK, Stottmeier KD, Sherman IH, McCabe WR. Mycobacte-
rial cervical lymphadenopathy. Relation of etiologic agent to age.
JAMA. 1984;251:1286–8. DOI: 10.1001/jama.251.10.1286
7. Lindeboom JA, Prins JM, Bruijnesteijn van Coppenraet ES, Linde-
boom R, Kuijper EJ. Cervicofacial lymphadenitis in children caused
by Mycobacterium haemophilum. Clin Infect Dis. 2005;41:1569–75.
DOI: 10.1086/497834
8. Tortoli E. Impact of genotypic studies on mycobacterial tax-
onomy: the new mycobacteria of the 1990s. Clin Microbiol Rev.
2003;16:319–54. DOI: 10.1128/CMR.16.2.319-354.2003
9. Ellison E, Lapuerta P, Martin SE. Fine needle aspiration diagnosis of
mycobacterial lymphadenitis. Sensitivity and predictive value in the
United States. Acta Cytol. 1999;43:153–7.
10. Lindeboom JA, Kuijper EJ, Prins JM, Bruijnesteijn van Coppenraet
ES, Lindeboom R. Tuberculin skin testing is useful in the screen-
ing for nontuberculous mycobacterial cervicofacial lymphadenitis in
children. Clin Infect Dis. 2006;43:1547–51. DOI: 10.1086/509326
11. Lindeboom JA, Kuijper EJ, Prins JM, Bruijnesteijn van Coppenraet
ES, Lindeboom R, Prins JM. Surgical excision versus antibiotic
treatment for nontuberculous mycobacteria cervical lymphadenitis
in children: a multicenter, randomized, controlled trial. Clin Infect
Dis. 2007;44:1057–64. DOI: 10.1086/512675
12. Petitjean G, Fluckiger U, Schären S, Laifer G. Vertebral osteomyeli-
tis caused by non-tuberculous mycobacteria. Clin Microbiol Infect.
2004;10:951–3. DOI: 10.1111/j.1469-0691.2004.00949.x
13. Elsayed S, Read R. Mycobacterium haemophilum osteomyelitis:
case report and review of the literature. BMC Infect Dis. 2006;6:70.
DOI: 10.1186/1471-2334-6-70
14. Zenone T, Boibieux A, Tigaud S, Fredenucci JF, Vincent V, Chidiac C,
et al. Non-tuberculous mycobacterial tenosynovitis: a review. Scand
J Infect Dis. 1999;31:221–8. DOI: 10.1080/00365549950163482
15. Theodorou DJ, Theodorou SJ, Kakitsubata Y, Sartoris DJ, Resnick
D. Imaging characteristics and epidemiological features of atypical
mycobacterial infections involving the musculoskeletal system. AJR
Am J Roentgenol. 2001;176:341–9.
16. Casal M, Casal MM; Spanish Group of Mycobacteriology. Multi-
center study of incidence of Mycobacterium marinum in humans in
Spain. Int. J Tuberc Lung Dis. 2001;5:197–9.
17. Hess CL, Wolock BS, Murphy MS. Mycobacterium marinum infec-
tions of the upper extremity. Plast Reconstr Surg. 2005;115:55e–9e.
DOI: 10.1097/01.PRS.0000153197.64808.B9
18. Edelstein H. Mycobacterium marinum skin infections. Report of 31
cases and review of the literature. Arch Intern Med. 1994;154:1359–
64. DOI: 10.1001/archinte.154.12.1359
19. Aubry A, Chosidow O, Caumes E, Robert J, Cambau E. Sixty-three
cases of Mycobacterium marinum infection: clinical features, treat-
ment, and antibiotic susceptibility of causative isolates. Arch Intern
Med. 2002;162:1746–52. DOI: 10.1001/archinte.162.15.1746
Emerging Infectious Diseases • www.cdc.gov/eid • Vol. 15, No. 9, September 2009 1357

SYNOPSIS
20. Ang P, Rattana-Apiromyakij N, Goh CL. Retrospective study
of Mycobacterium marinum skin infections. Int J Dermatol.
2000;39:343–7. DOI: 10.1046/j.1365-4362.2000.00916.x
21. Bartralot R, Pujol RM, García-Patos V, Sitjas D, Martín-Casabona
N, Coll P, et al. Cutaneous infections due to nontuberculous myco-
bacteria: histopathological review of 28 cases. Comparative study
between lesions observed in immunosuppressed patients and normal
hosts. J Cutan Pathol. 2000;27:124–9. DOI: 10.1034/j.1600-0560
.2000.027003124.x
22. Clinical and Laboratory Standards Institute. Laboratory detection
and identication of mycobacteria: approved guideline. CLSI docu-
ment M48-A. Wayne (PA): The Institute; 2008.
23. Cloud JL, Neal H, Rosenberry R, Turenne CY, Jama M, Hillyard
DR, et al. Identication of Mycobacterium spp. by using a com-
mercial 16S ribosomal DNA sequencing kit and additional sequenc-
ing libraries. J Clin Microbiol. 2002;40:400–6. DOI: 10.1128/
JCM.40.2.400-406.2002
24. Bråbäck M, Riesbeck K, Forsgren A. Susceptibilities of Mycobacte-
rium marinum to gatioxacin, gemioxacin, levooxacin, linezolid,
moxioxacin, telithromycin, and quinupristin-dalfopristin (Syner-
cid) compared to its susceptibilities to reference macrolides and
quinolones. Antimicrob Agents Chemother. 2002;46:1114–6. DOI:
10.1128/AAC.46.4.1114-1116.2002
25. National Committee for Clinical Laboratory Standards. Susceptibil-
ity testing of mycobacteria, nocardia, and other aerobic actinomycet-
es. Approved standard M24-A. Wayne (PA): The Committee; 2003.
26. World Health Organization. Buruli ulcer disease. Mycobacterium
ulcerans infection: an overview of reported cases globally. Wkly
Epidemiol Rec. 2004;79:194–200.
27. Amofah G, Bonsu F, Tetteh C, Okrah J, Asamoa K. Buruli ul-
cer in Ghana: results of a national case search. Emerg Infect Dis.
2002;8:167–70.
28. Marsollier L, Severin T, Aubry J, Merritt RW, Saint Andre JP,
Legras P, et al. Aquatic snails, passive hosts of Mycobacterium ul-
cerans. Appl Environ Microbiol. 2004;70:6296–8. DOI: 10.1128/
AEM.70.10.6296-6298.2004
29. Debacker M, Aguiar J, Steunou C, Zinsou C, Meyers WM, Scott
JT, et al. Mycobacterium ulcerans disease: role of age and gender
in incidence and morbidity. Trop Med Int Health. 2004;9:1297–304.
DOI: 10.1111/j.1365-3156.2004.01339.x
30. Guarner J, Bartlett J, Whitney EA, Raghunathan PL, Stienstra Y,
Asamoa K, et al. Histopathologic features of Mycobacterium ulcer-
ans infection. Emerg Infect Dis. 2003;9:651–6.
31. Ellen DE, Stienstra Y, Teelken MA, Dijkstra PU, van der Graaf WT,
van der Werf TS. Assessment of functional limitations caused by
Mycobacterium ulcerans infections: towards a Buruli ulcer func-
tional limitation score. Trop Med Int Health. 2003;8:90–6. DOI:
10.1046/j.1365-3156.2003.00976.x
32. Ross BC, Marino L, Oppedisano F, Edwards R, Robins-Browne RM,
Johnson PD. Development of a PCR assay for rapid diagnosis of
Mycobacterium ulcerans infection. J Clin Microbiol. 1997;35:1696–
700.
33. Wansbrough-Jones M, Phillips R. Buruli ulcer: emerging from
obscurity. Lancet. 2006;367:1849–58. DOI: 10.1016/S0140-6736
(06)68807-7
34. Etuaful S, Carbonnelle B, Grosset J, Lucas S, Horseld C, Phillips
R, et al. Efcacy of the combination rifampin-streptomycin in pre-
venting growth of Mycobacterium ulcerans in early lesions of Buruli
ulcer in humans. Antimicrob Agents Chemother. 2005;49:3182–6.
DOI: 10.1128/AAC.49.8.3182-3186.2005
35. Brown-Elliott BA, Wallace RJ Jr. Clinical and taxonomic status
of pathogenic nonpigmented late-pigmenting rapidly growing my-
cobacteria. Clin Microbiol Rev. 2002;15:716–46. DOI: 10.1128/
CMR.15.4.716-746.2002
36. Hall-Stoodley L, Stoodley P. Biolm formation and dispersal and the
transmission of human pathogens. Trends Microbiol. 2005;13:7–10.
DOI: 10.1016/j.tim.2004.11.004
37. Wallace RJ Jr, Brown BA, Grifth DE. Nosocomial outbreaks/pseu-
do-outbreaks caused by nontuberculous mycobacteria. Annu Rev
Microbiol. 1998;52:453–90. DOI: 10.1146/annurev.micro.52.1.453
38. Uslan DZ, Kowalski TJ, Wengenack LW, Virk A, Wilson JW. Skin
and soft tissue infections due to rapidly growing mycobacteria. Arch
Dermatol. 2006;142:1287–92. DOI: 10.1001/archderm.142.10.1287
39. Winthrop KL, Abrams M, Yakrus M, Schwartz I, Ely J, Gillies D,
et al. An outbreak of mycobacterial furunculosis associated with
footbaths at a nail salon. N Engl J Med. 2002;346:1366–71. DOI:
10.1056/NEJMoa012643
40. Turenne CY, Tschetter L, Wolfe J, Kabani A. Necessity of quality-
controlled 16S rRNA gene sequence databases: identifying nontu-
berculous Mycobacterium species. J Clin Microbiol. 2001;39:3637–
48. DOI: 10.1128/JCM.39.10.3638-3648.2001
Address for correspondence: Claudio Piersimoni, Department of Clinical
Microbiology, United Hospitals, via Conca 71, I-60020 Ancona, Italy;
email: [email protected]
1358 Emerging Infectious Diseases • www.cdc.gov/eid • Vol. 15, No. 9, September 2009
